YOU ARE LEARNING:
What Fusion Is
What Fusion Is
Nuclear fusion is when two smaller nuclei join together into a larger, heavier nucleus, releasing lots of energy in the process.
Nuclear fission is when a very large nucleus splits into smaller nuclei. We can think of nuclear fusion as the opposite. So what do you think nuclear fusion is?

Which reaction do you think releases more energy?

So nuclear fission is when a nucleus splits. Nuclear fusion is when two nuclei join together. Nuclear fusion releases absolutely tremendous amounts of energy! But it also requires enormous amounts of energy, because the nuclei have to smash together hard enough to actually fuse together.
So how does nuclear fusion really happen? To understand that, first remember what makes up an atom.
The nucleus of an atom is made up of which two subatomic particles?

When atoms of the same element, for example two carbon atoms, have a different number of neutrons in the nucleus, we call them ____________.

These are three isotopes of hydrogen
Each isotope has 1 proton and 1 electron, but they all have a different number of neutrons. The one with one neutron is called deuterium, and the one with two neutrons is called tritium.
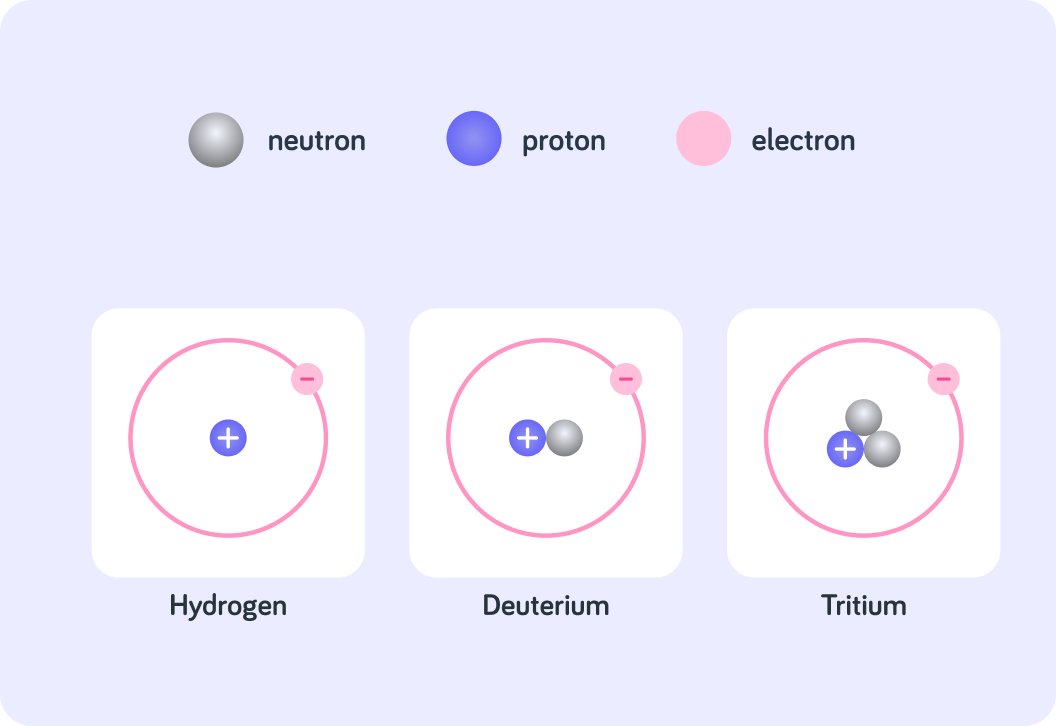
The nuclide notation of deuterium is 12H. How would you write the nuclide notation of tritium? Use the fraction tool like so ??H .


Nuclear fusion requires and releases enormous amounts of energy, so where do you think it might occur naturally?
A) At the centre of the Earth B) In the core of a star like our Sun C) In nuclear power plants
Answer A, B or C.


Inside the Sun, hydrogen nuclei fuse in pairs and become another element. Which element will that be?


So two hydrogen nuclei fuse into a helium nucleus
This releases tremendous amounts of energy! It also emits a neutron.

There are different isotopes of helium, just like there are different isotopes of hydrogen. One example of a helium isotope is 24He . What is the mass of this helium isotope?


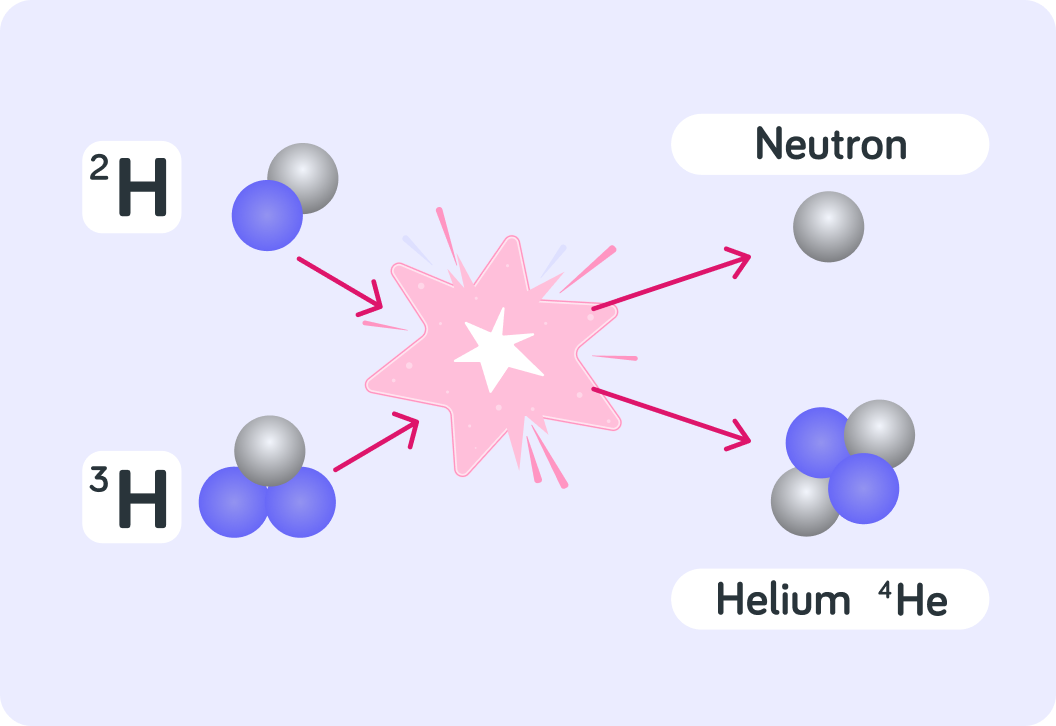
So if two hydrogen isotopes inside the Sun fuse to form the isotope 24He , which two hydrogen isotopes were they? Remember that the reaction emits a neutron.
A) Deuterium and hydrogen B) Deuterium and tritium C) Tritium and hydrogen


This shows the reaction of two hydrogen isotopes fusing into a helium isotope.
The reaction releases huge amounts of energy and a neutron is emitted.
Now, in nuclear fission, the products are radioactive because the products are unstable nuclei. Are the products of nuclear fusion also radioactive? Answer yes or no.


When the small hydrogen nuclei fuse together and loose a neutron in the process, they become a larger and stable helium nucleus.
The helium nucleus has a balanced number of protons and neutrons for its size, so it does not decay and does not emit radiation.

Stars like our Sun are powered by nuclear fusion reactions at their core, which releases a tremendous amount of energy. It's the fusion at the Sun's core that makes it so remarkably ____________ and ____________.

You can select multiple answers
Throughout the life cycle of a star, fusion in the core produces heavier and heavier elements. For example, two hydrogen atoms (atomic number 1) fuse to become a helium atom (atomic number 2).
When two helium nuclei fuse, they form beryllium. So what is beryllium's atomic number?

We can't induce nuclear fusion on Earth very easily, so what is it about a star that makes fusion possible there?
What is the most obvious property of the Sun which it would be a challenge for us to recreate here on Earth? The Sun is very very _____!

Nuclear fusion requires extremely high temperatures!
The temperature at the Sun's core is around 15,000,000° C.
Now, imagine you had two balls of clay and wanted to combine them into one. To do that, you would squeeze them together, so you would apply ____________. (It begins with a "p").

It also requires pressure to bring two atoms close together
And it requires absolutely unbelievable amounts of pressure to make two atoms actually fuse.
That pressure is present inside stars
Stars are very big and heavy, so they have a lot of gravitational force. It's very hard for scientists to replicate that amount of pressure here on Earth.
Scientists have worked for many years to try to create a nuclear fusion reactor on Earth, and they are still going. The fuel would be hydrogen. Which substance do you think they are planning on extracting the hydrogen from?

Some of these statements refer to nuclear fusion, and some refer to nuclear fission. Pick all the options that are true about nuclear fusion.

You can select multiple answers
Summary
Nuclear fusion is when two smaller nuclei join together into a larger, heavier nucleus.
For example when two hydrogen nuclei fuse and become a helium nucleus.
The product of nuclear fusion is not radioactive like the products of nuclear fission.
This is because the process emits a neutron, so that the product nucleus is in fact stable. That means it does not decay and does not emit radiation.
This reaction releases tremendous amounts of energy!
Much more energy even than nuclear fission.
However, it also requires extreme amounts of temperature and pressure to induce
This is why we still don't have nuclear fusion reactors on Earth. We only have nuclear fission reactors.
However, scientists are working on how to make a nuclear reactor work on Earth
They want to use the hydrogen in water as fuel for nuclear fusion.
