YOU ARE LEARNING:
Carbon Dating
Carbon Dating
The radioactive isotope carbon-14 can be used to find the age of archaeological specimens.
Imagine you find an old fossil
You can use carbon dating to find out how old it actually is by looking at the amount of carbon-14 in the specimen.

Carbon-14 is an unstable isotope which means it will ___________ over time.
A) fuse B) collapse C) decay


The time it takes for the amount of a radioactive isotope like carbon-14 to reduce by 50% is called its ______________.


In this fossil, there is only 12.5% of the original amount of carbon-14 left, so how many times has the original amount halved since the now fossilised animal died?

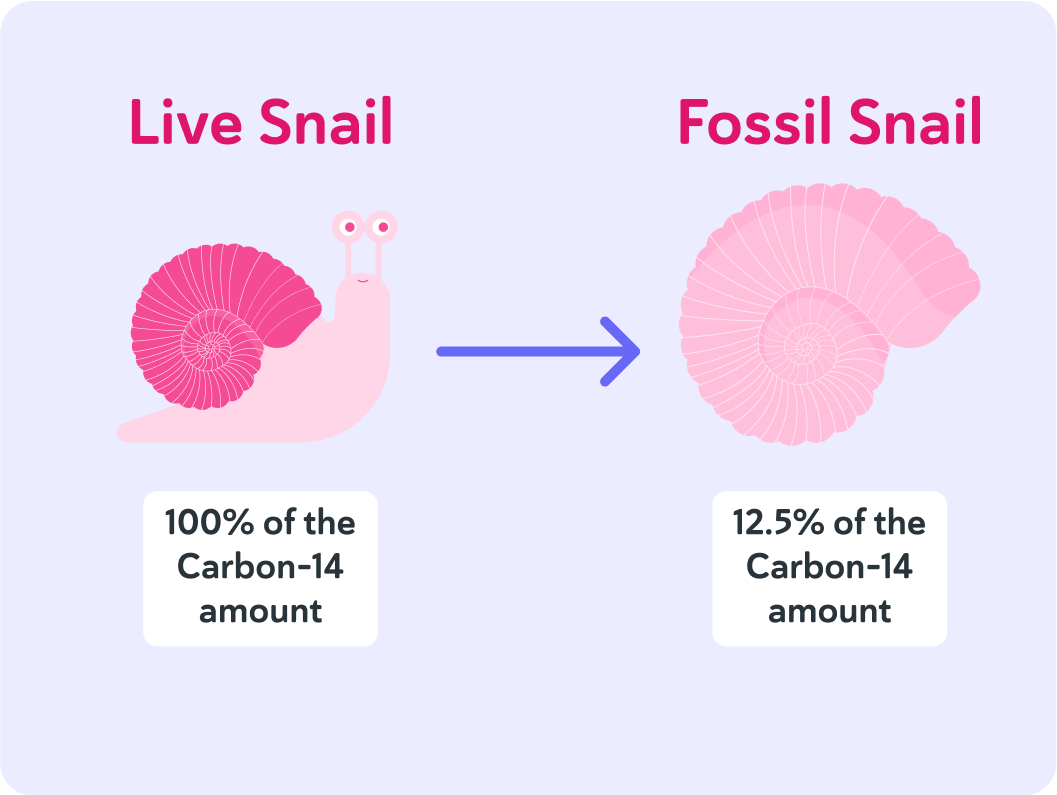
The half-life of carbon-14 is 5,730 years, and it's been 3 half-lives since the animal died. So how long ago is that?

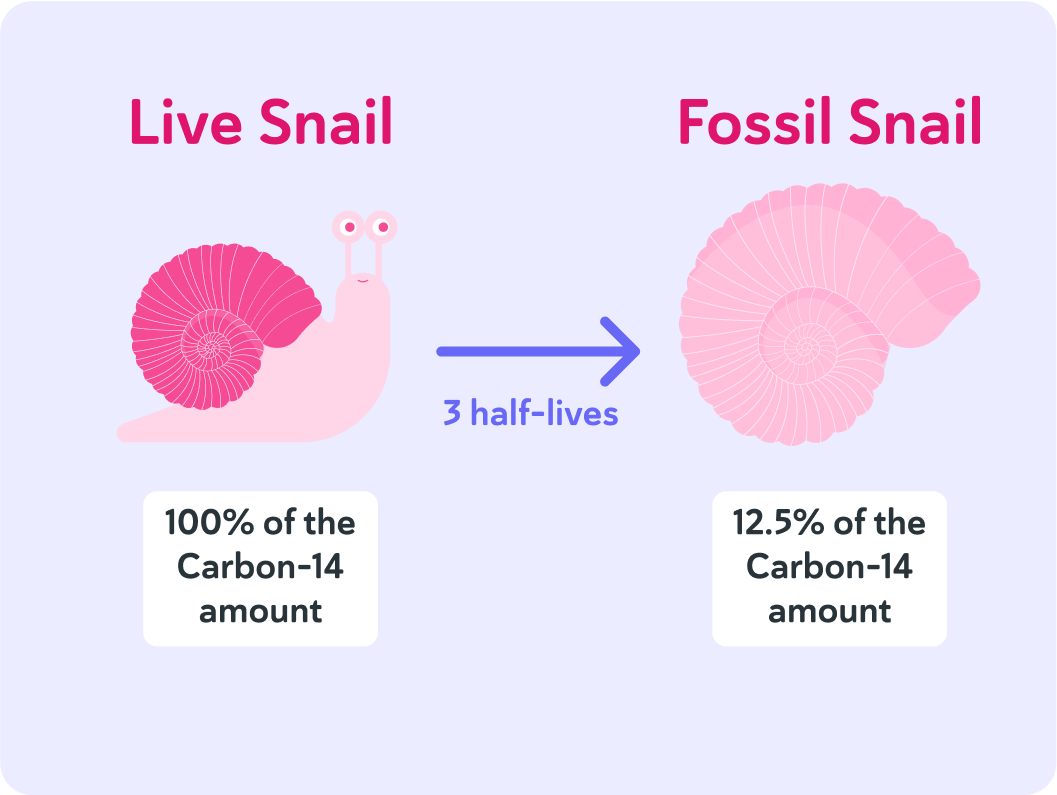
So the age of the fossil can be calculated by looking at how much carbon-14 it contains, compared to how much it had when it died.
If that is 12.5%, that equals 3 half-lives.
The half-life of carbon-14 is 5,730 years, so that means it has been 5,730×3=17,190 years since the specimen was alive.
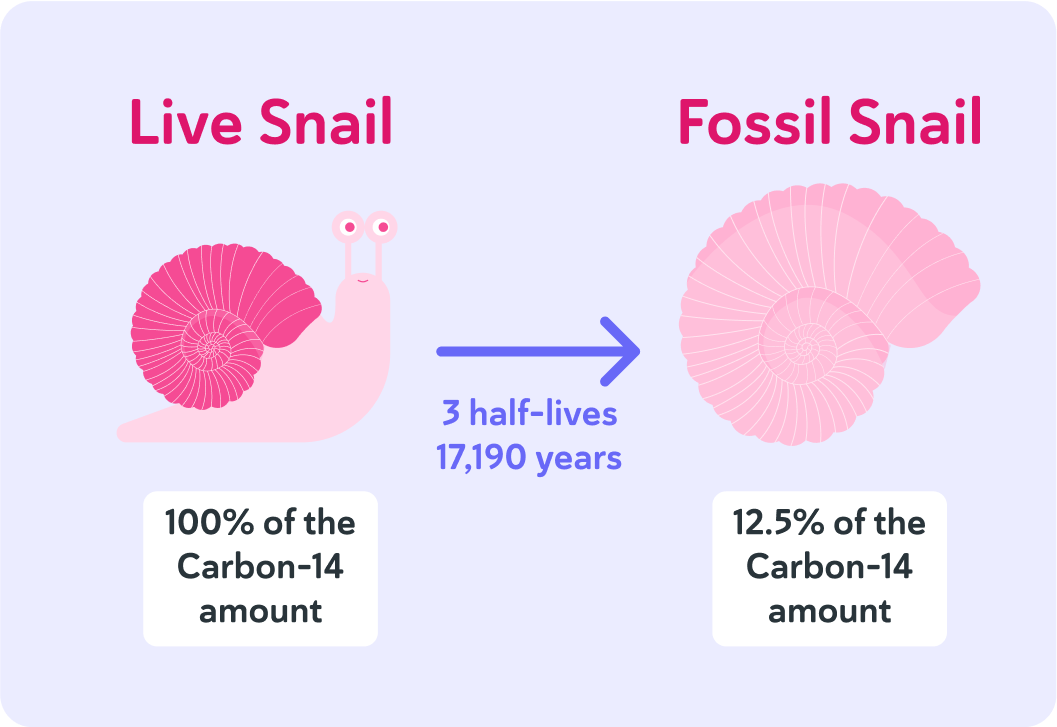
But how does carbon-14 get into living things in the first place? And why does the amount of carbon-14 in the specimen decline only after the specimen has died?
Which organisms take in carbon dioxide and turn it into food?

Plants take in carbon dioxide and turn it into energy for themselves and release oxygen to the atmosphere. This process is called ________________.

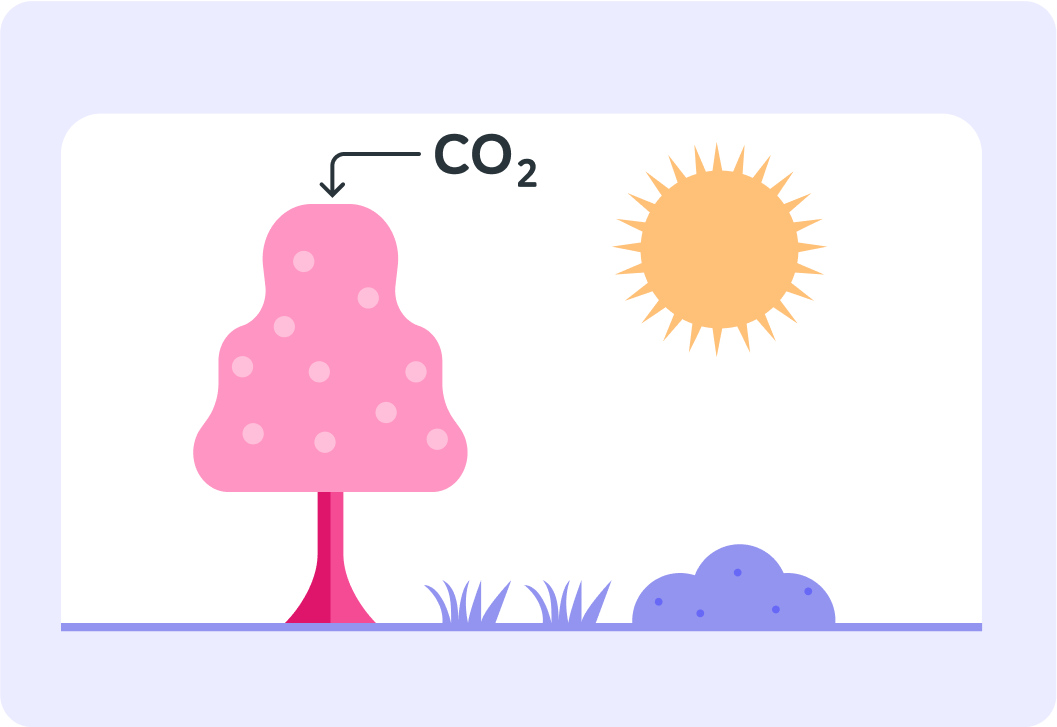
Both normal carbon and carbon-14 in the atmosphere can form carbon dioxide and get absorbed by plants via photosynthesis
So the proportion of carbon-14 in the living plant is the same as the proportion of carbon-14 in the atmosphere.

Now, does a dead plant perform photosynthesis? Answer yes or no.


When the plant dies, it no longer performs photosynthesis and will not take in any more carbon-14
The carbon-14 in the plant will start to decay. So over time, the amount of carbon-14 in the dead plant will decline.

How does carbon-14 make it into animals, then?
You know that carbon-14 makes it into plants via photosynthesis.
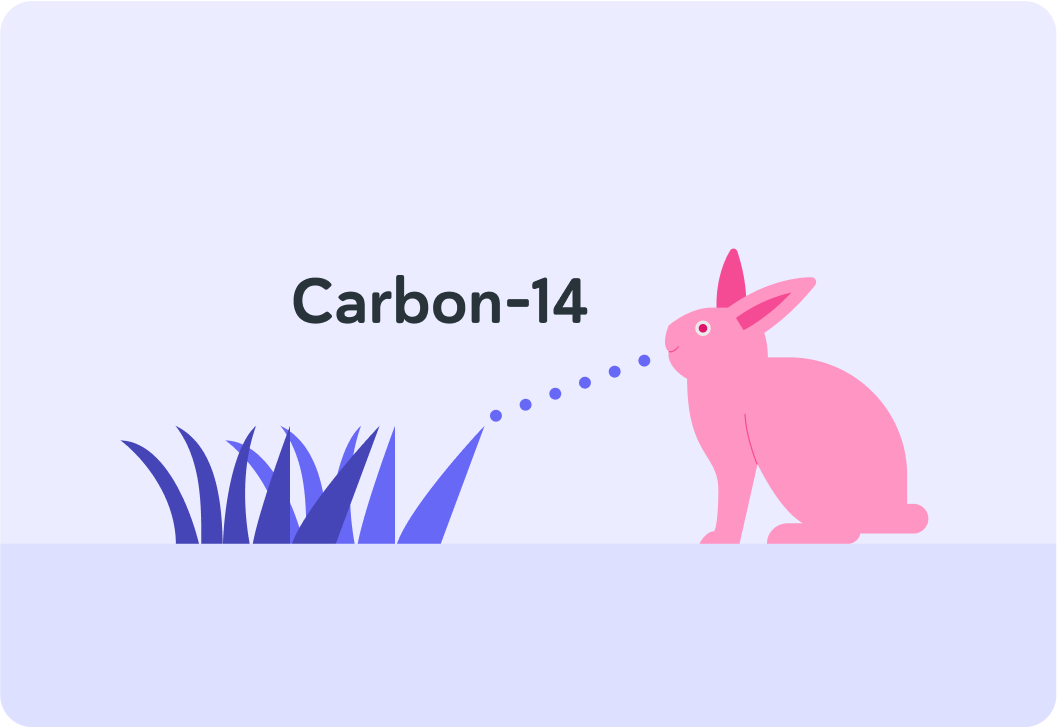
How do plants make it into animals?
A) Plants get eaten by animals. B) Plants get rubbed into animals. C) Plants don't get onto animals.


So carbon-14 gets into animals when animals eat plants
Animals that don't eat plants usually eat other animals that do eat plants.
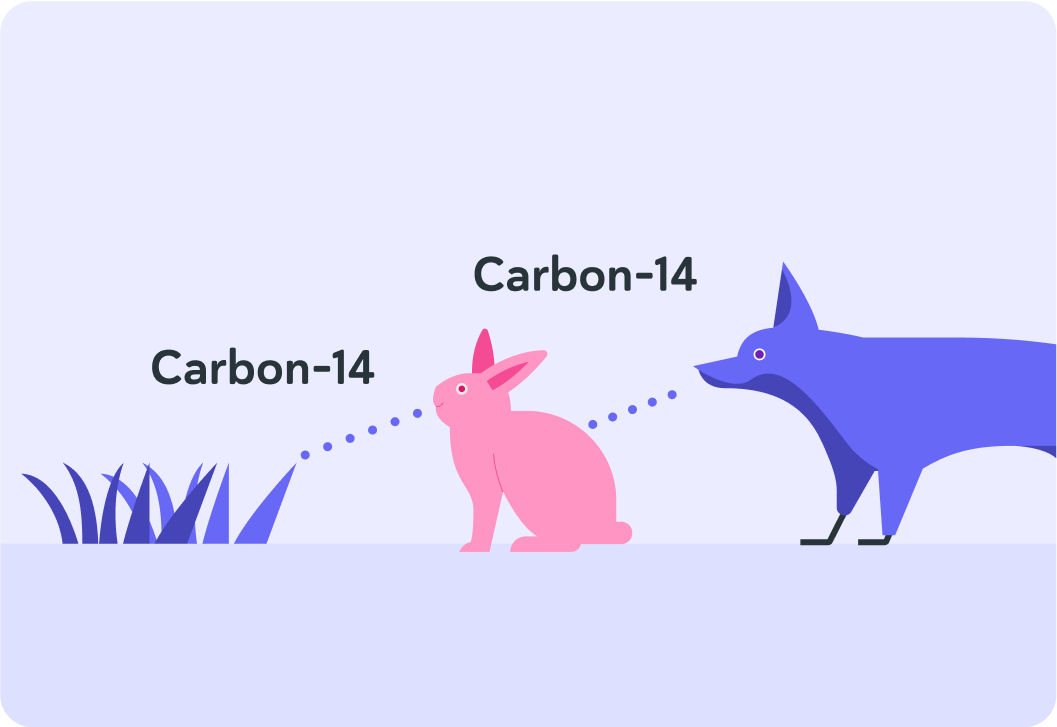
What do we call this chain, where plants get eaten by animals and animals get eaten by other animals?


So carbon-14 enters living organisms via the food chain
When organisms die, they no longer eat, so they no longer take in any more carbon-14.

True or false? You can only use carbon dating on specimens that used to be alive.

Which of the following materials do you think carbon dating can be used on?

You can select multiple answers
To recap
You use carbon dating to decide how old a specimen is, for example a fossil
Essentially, you decide how long ago it is since the specimen died.
Carbon-14 enters living things via the food chain
Plants take in carbon-14 from the atmosphere during photosynthesis. Plant eating animals then eat the plants. Finally, other animals eat the plant eaters.
When living things die, they stop eating and photosynthesising, so they stop taking in carbon-14
The amount of carbon-14 that was in the organisms will start to decay, which means the amount of it will decline.
So when you decide how old a specimen is, you measure the amount of carbon-14 in the specimen
You then compare that to the amount of carbon-14 in the atmosphere.
For example, the amount of carbon-14 in the specimen might be only 12.5% of the amount that is in the atmosphere
That means it has been 3 half-lives since the specimen died.
The half-life of carbon-14 is 5,730 years
So if the amount of carbon-14 is only 12.5% of the amount that is in the atmosphere, that means it's 17,190 years since the specimen died.
We can only use carbon dating to date specimens that used to be alive
We can't date for example metal, because metal was never part of a food chain, so it never took in carbon-14.
By the way
It is actually only a very small amount of carbon atoms in the atmosphere and in living things that are carbon-14 isotopes - only about 1 in every 850 billion carbon atoms!
Where does carbon-14 come from in the first place?
In very short, it forms in the Earth's upper atmosphere from nitrogen-14.
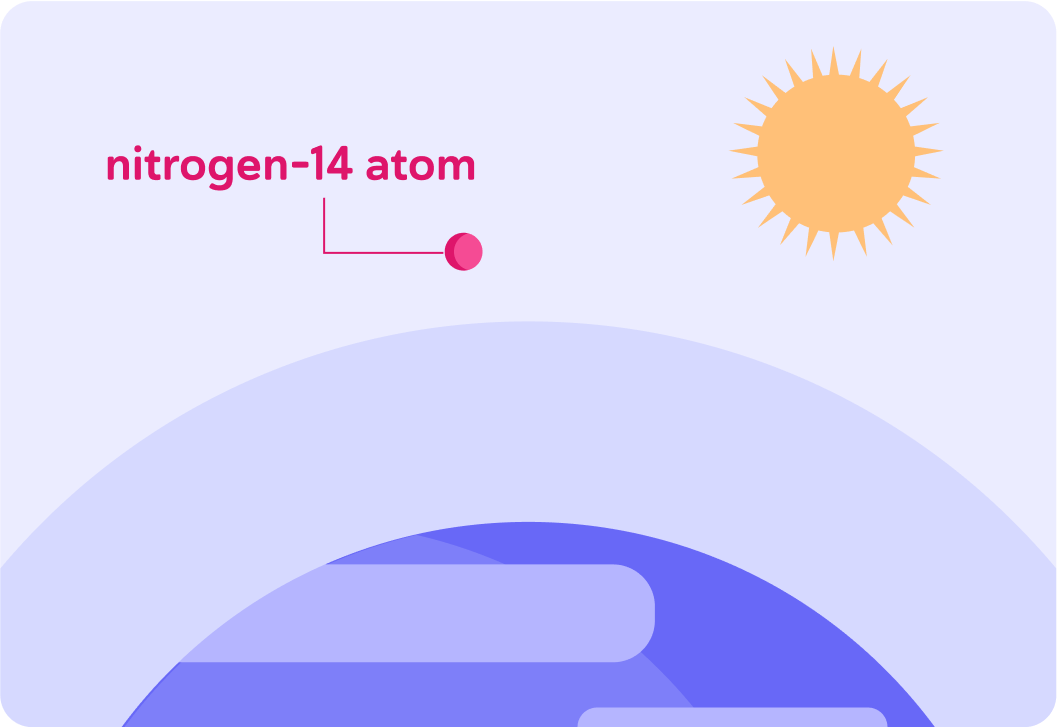
Nitrogen-14 in the Earth's upper atmosphere has a mass of 14 and atomic number 7. What is its nuclide notation?
A) 147N B) 77N C) 714N


Now, cosmic rays from the Sun collide with atoms on the atmosphere and produce energetic neutrons
These neutrons collide with the nitrogen atoms.
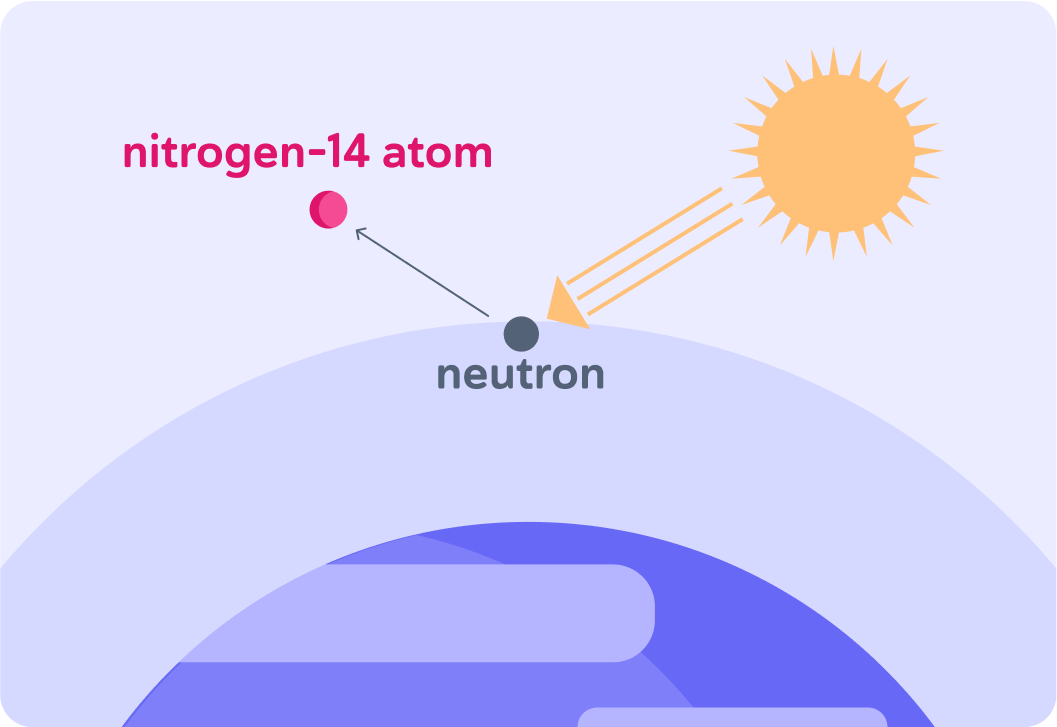
When these neutrons collide with the nitrogen atoms it makes them unstable, so the nitrogen atoms ________.
A) decay B) fuse C) collapse


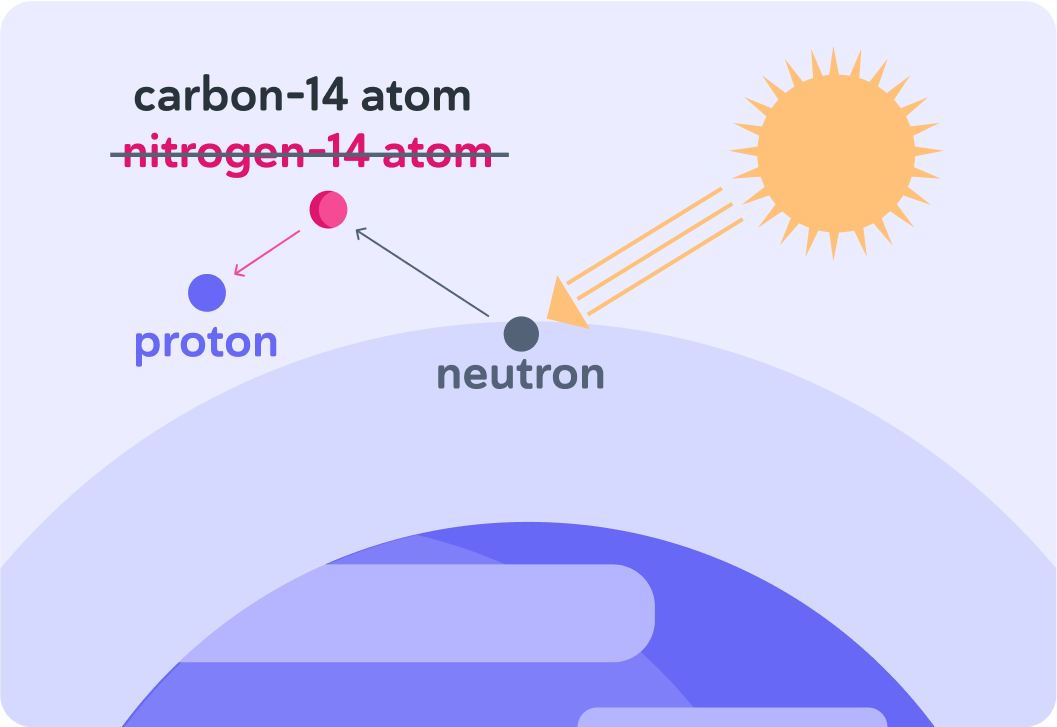
When the nitrogen atom decays, it emits a proton.
So the nitrogen-14 atoms turn into a carbon-14 isotopes.
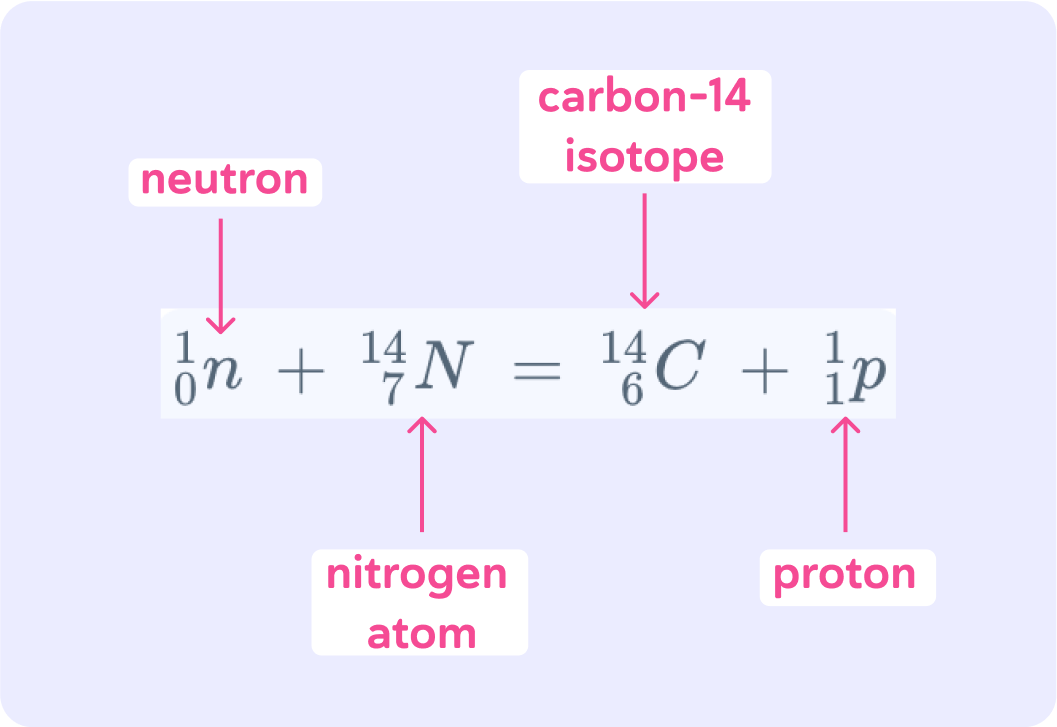
This nuclear equation sums up the process
A neutron collides with a nitrogen atom which makes it unstable. When the nitrogen atom has emitted a proton, what's left is a carbon-14 isotope.
What happens when carbon-14 decays?
In its most stable form, carbon has a mass of 12. This means that carbon-14 is an unstable ____________ of carbon-12.

Carbon-14 undergoes beta minus decay. That means that it emits a fast moving ___________.

Now, when nitrogen-14 emitted a proton it turned into carbon-14. When carbon-14 decays and emits an electron, it turns back into ___________.

Carbon-14 is created about as quickly as it turns back into nitrogen-14. What does that mean for the amount of carbon-14 present in the atmosphere?

There is a more or less fixed amount of carbon-14 in the atmosphere
That is why plants and animals have the same proportion of carbon-14 inside them the moment they die as the proportion in the atmosphere.
Uses and limitations of carbon dating
What is the most obvious limitation of carbon dating?

Carbon dating can't be used on things that were never alive
This is because things that were never alive have never taken in carbon from the atmosphere. For example, brick, rock and metal have never been alive, so they don't contain any carbon-14.
Now, imagine an archaeological specimen that is 60,000 years old. This means that over 10 half-lives have gone by since the specimen died. How much carbon-14 would be present in the specimen?

The amount of carbon-14 in samples is very small
After 9 or 10 half-lives the amount of radioactivity which is emitted by the sample is far too small for an accurate count rate to be measured.
So carbon dating can't be used to date samples which are over 50,000 years old.
Finally, carbon dating is based on an assumption
We assume that the amount of carbon-14 present in the atmosphere in the past is the same as today.
If this assumption is not correct then carbon dating does not find the correct age for specimens.
Summary! Carbon-14 can be used to determine old an archaeological specimen is
You measure the amount of carbon-14 left in the specimen, and compare it to the percentage of carbon-14 in the atmosphere. You then calculate how many half-lives have passed. Finally, you multiply that by carbon-14's half-life, which is 5,730 years.

Carbon-14 gets into living things via the food chain
First it enters plants in photosynthesis. Then animals eat plants or other animals.

Carbon-14 gets formed from nitrogen-14 in the Earth's upper atmosphere
Neutrons collide with nitrogen-14, which makes it emit a proton and turns it into carbon-14.

Carbon-14 is an unstable isotope that undergoes beta minus decay
That means one of its neutrons turns into a proton and emits an electron, so it turns back into nitrogen-14.

Nitrogen-14 turns into carbon-14 at about the same rate as carbon-14 turns back into nitrogen-14
So there is always pretty much the same amount of carbon-14 in the atmosphere. And it's not very much! There is only about 1 carbon-14 isotope for every 850 billion carbon atoms in the atmosphere.

Carbon dating does have a few constraints though
We can only use carbon dating to determine the age of specimens that used to be alive, for example plant, animal or human remains (skin, fur, bone, wood, seed and things like cotton and wool). We cannot use it on for example bricks, rock or metal.

We also cannot use carbon dating when the specimen is older than 50,000 years
If the specimen is older than that the amount of carbon-14 left in it is so small that we can't measure it precisely.

Finally, carbon dating rests on an assumption
We assume that the amount of carbon-14 in the atmosphere when the specimen was alive is the same as the amount of carbon-14 in the atmosphere today.

