YOU ARE LEARNING:
Fission of U-235

Fission of U-235
Uranium-235 is a naturally radioactive element commonly used as fuel for nuclear fission in power plants.
Nuclear fission is the...

Nuclear power plants use nuclear reactors to release energy through the process of nuclear fission. This is the splitting of a nucleus. Don't confuse it with nuclear fusion, where two nuclei get fused together.
For nuclear fission to occur, we need a large and heavy nucleus. Which element do you think we most commonly use as fuel in nuclear fission?

We use different isotopes of uranium as fuel for nuclear fission. Specifically, we use U-238 and U-235. The numbers 238 and 235 refer to their atomic masses, so how are they different from each other?

The image shows the isotopes U-235 and U-238. How many protons do both isotopes have in their nucleus? You can find this number in the nuclide notation.

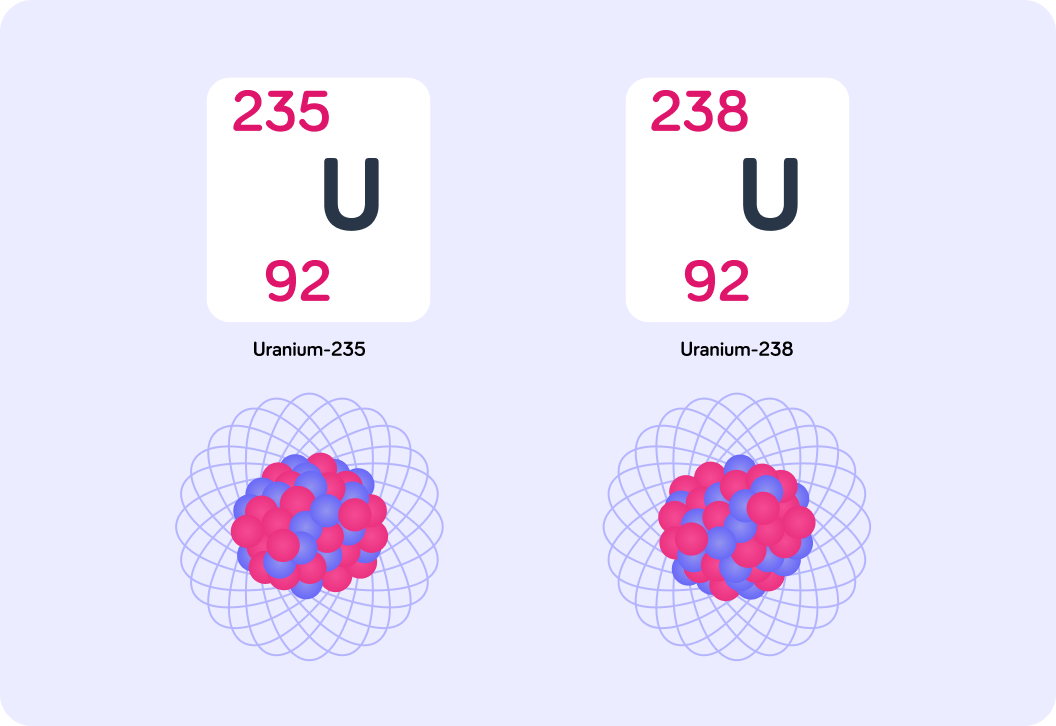
So U-235 has 92 protons. If the atomic mass is the sum of the number of protons and the number of neutrons, how many neutrons does U-235 have in its nucleus?


How many neutrons does U-238 have in its nucleus?


Uranium is naturally radioactive because its nucleus is unstable.
It is the imbalance of protons and neutrons in its nucleus that makes it unstable. In the case of uranium there are too many neutrons compared to protons.

Where there are too few or too many neutrons in a nucleus, it is unstable. This means that it will decay and emit radiation.
In a nuclear reactor, a uranium-235 nucleus is struck by a neutron. When the neutron is absorbed into the nucleus, it becomes uranium-236. Now, the nucleus is...

The image shows the process of the nuclear fission of U-235 in a nuclear reactor. Particle A bombards the nucleus and is absorbed by it. What type of particle is A?

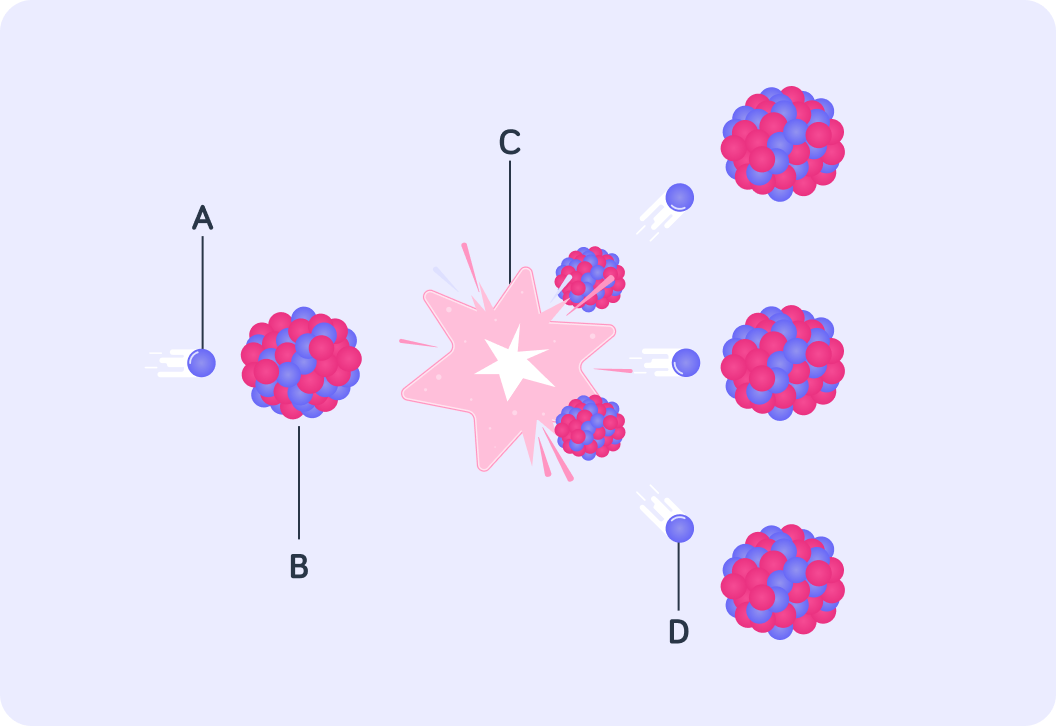
The neutron is absorbed by the U-235 nucleus making it violently unstable. This causes the nucleus to split. What does this release? What does should label C say in the diagram?


Energy is not the only product of this reaction. Two smaller nuclei are formed, and two or three neutrons are also emitted. These neutrons are released at very high speeds, so what type of energy do they store?


So each fission event can cause further fission events. We call this a chain reaction. What best describes how a chain reaction occurs?
A) The lighter nuclei that are formed split further. B) One or more of the emitted neutrons strike other U-235 nuclei. C) One or more of the emitted neutrons strike the lighter nuclei.
Answer A, B or C.


So fission is a form of radioactive decay where the nucleus splits into two or more parts.
When this happens naturally, it happens spontaneously and randomly. However, in a nuclear reactor it is induced by the bombardment of neutrons.

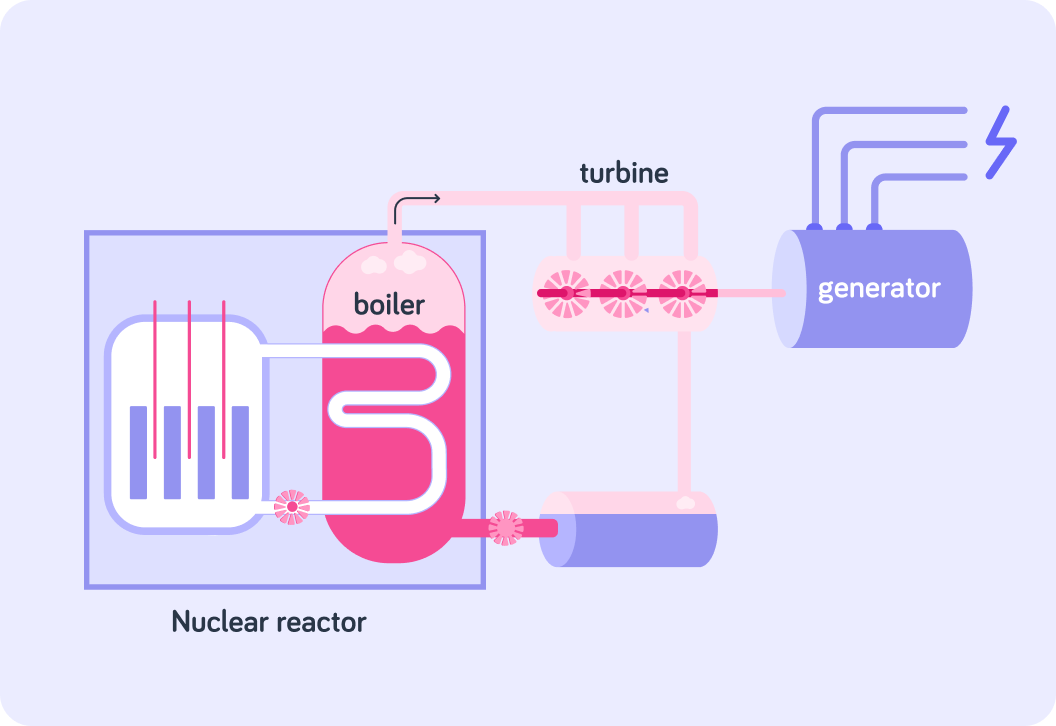
The energy released in this process is used to heat water.
The water then drives turbines that turn a generator to produce electricity. This is how a nuclear reactor in a power station produces electricity.
An uncontrolled fission chain reaction can be tremendously dangerous! In fact, this is how atomic bombs work. How do you think the process is controlled in a nuclear reactor?

When the uranium nucleus splits it emits radiation. The symbol used for this type of radiation is γ. It is a wave, rather than a particle. What do we call this type of radiation?

The smaller nuclei which U-235 splits into, are referred to as the products of nuclear fission. They are typically unstable, radioactive isotopes of elements like barium, krypton or strontium. How do you think these products behave once they are formed?

So the products of nuclear fission are radioactive and therefore dangerous. That is why we take special care to contain and dispose of the waste products safely.
