YOU ARE LEARNING:
The Formula for Density

The Formula for Density
You calculate a substance's density by dividing mass by volume.
The density of water VS. the density of lead
There is the same volume of water and lead in this image?

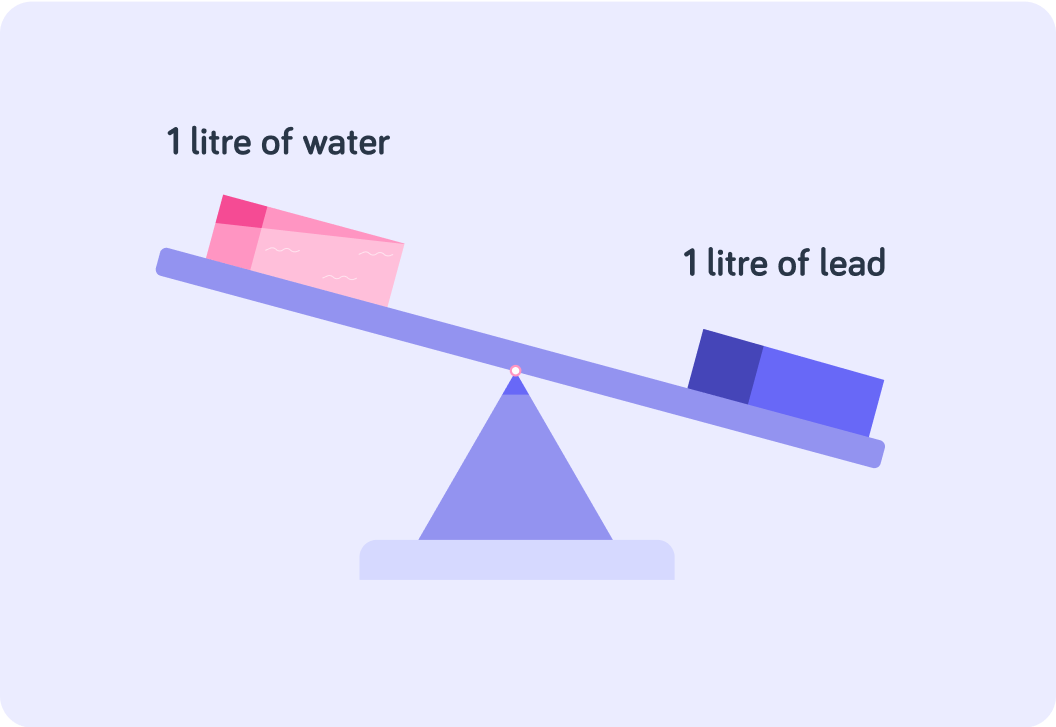
Does the lead and the water in this image have the same mass?


So the water and the lead have the same volume (1 litre), but different mass. Do you think water and lead have the same density?


Which substance is more dense?


The formula for density
First of all, 1 litre is the same as 1,000 cm3
So 1 litre of water or lead is the same as 1,000 cm3 of water or lead.
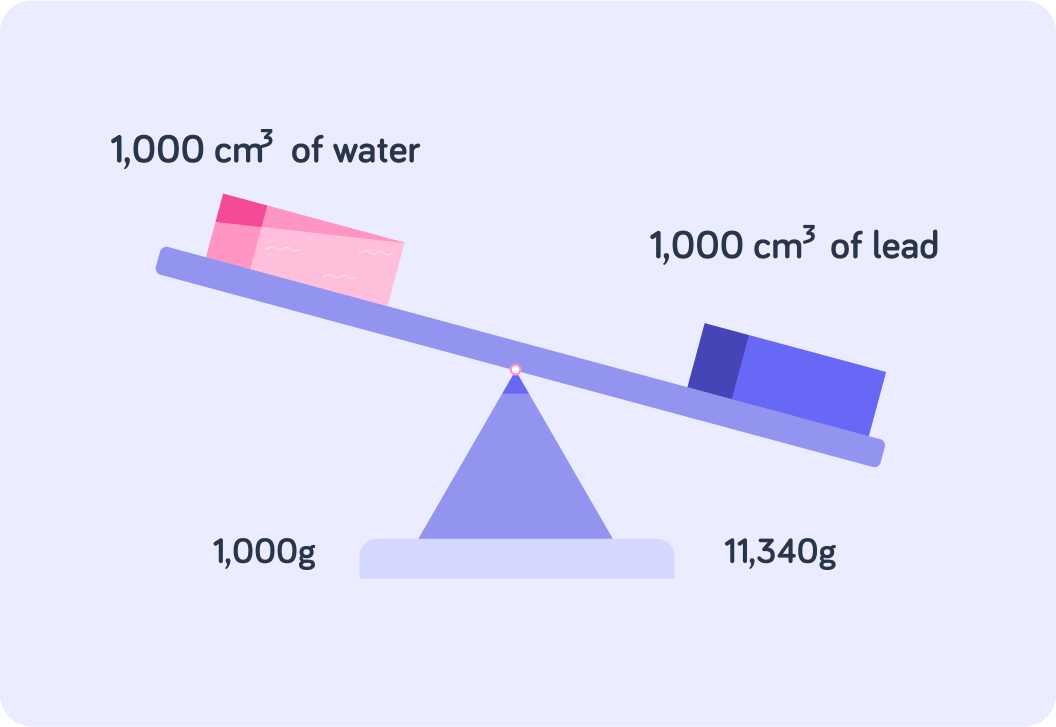
Have a look at the image. What is the mass of 1,000 cm3 of lead?


Now, the density of lead is 11.34 g/cm3. So how do you calculate the density of lead?


So you calculate the density of lead like this 1,000 cm311,340 g. So what is the generalised formula for calculating density?


What is the density of water in g/cm3?


Recap!
The density of an object is dependent on its volume and mass
For example, if you have two objects of the same volume, but one has more mass than the other, then the heavier object will be the denser one.

The formula for density is density=volumemass
The density of water is 1 g/cm3 The density of lead is 11.34 g/cm3

So one litre of lead is more than 11 kilos heavy
That is more than 11 times heavier than the same amount of water!

Units for density
g/cm3 is probably most common unit used for the density of substances on Earth. Which one of these options could we also use?

The unit kg/cm3 also contains a unit for mass and a unit for volume, so it is a density unit. However, we don't use it much, because it implies that an object is extremely _____________.

So the more common units for density are g/cm3 or kg/m3
We don't really use for example kg/cm3 about objects on Earth, because materials here just aren't so dense that they weigh several kilos per cubic centimetre!
A substance has mass 16 g and volume 8 cm3. What is the substance's density in g/cm3?

A substance has mass 240 kg and volume 6 m3. What is the substance's density in kg/m3?

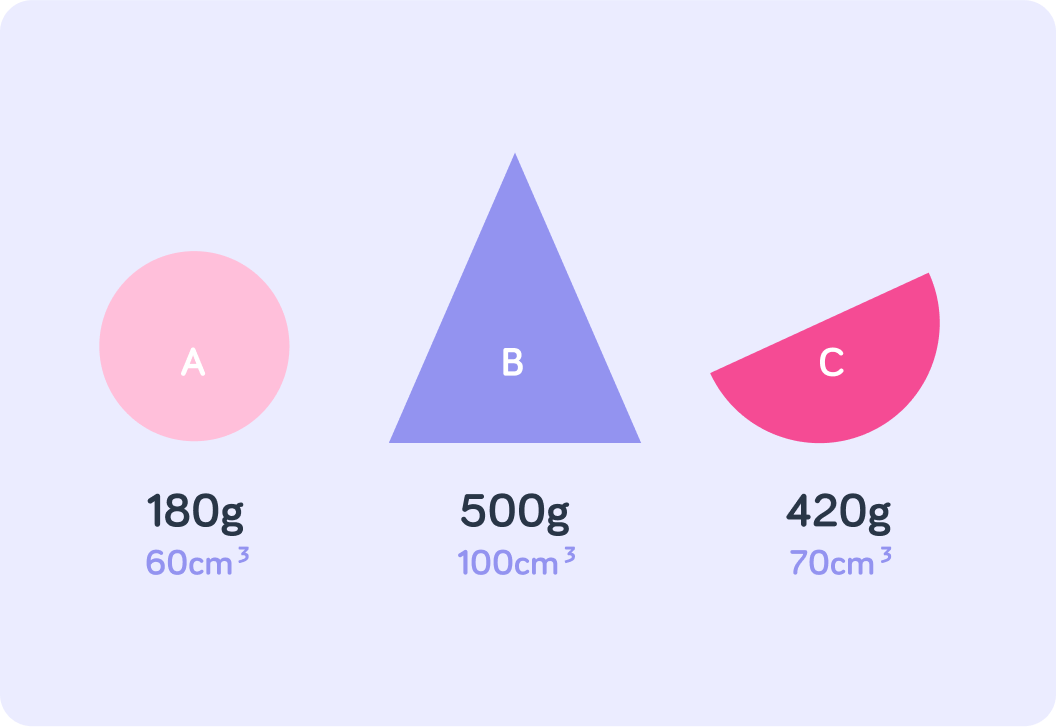
Which one of these objects is the most dense? Answer A, B or C.

Summary!
The formula for density is density=volumemass
This gives you units like g/cm3 or kg/m3

For example, here object C is denser than objects A and B
Object A has a density of 3 g/cm3
Object B has a density of 5 g/cm3
Object C has a density of 6 g/cm3

