YOU ARE LEARNING:
What Is Density?

What Is Density?
The density of an object is dependent on its mass and its volume.
Take a very good look at this image
The iron and the feathers have the same ** mass*.*

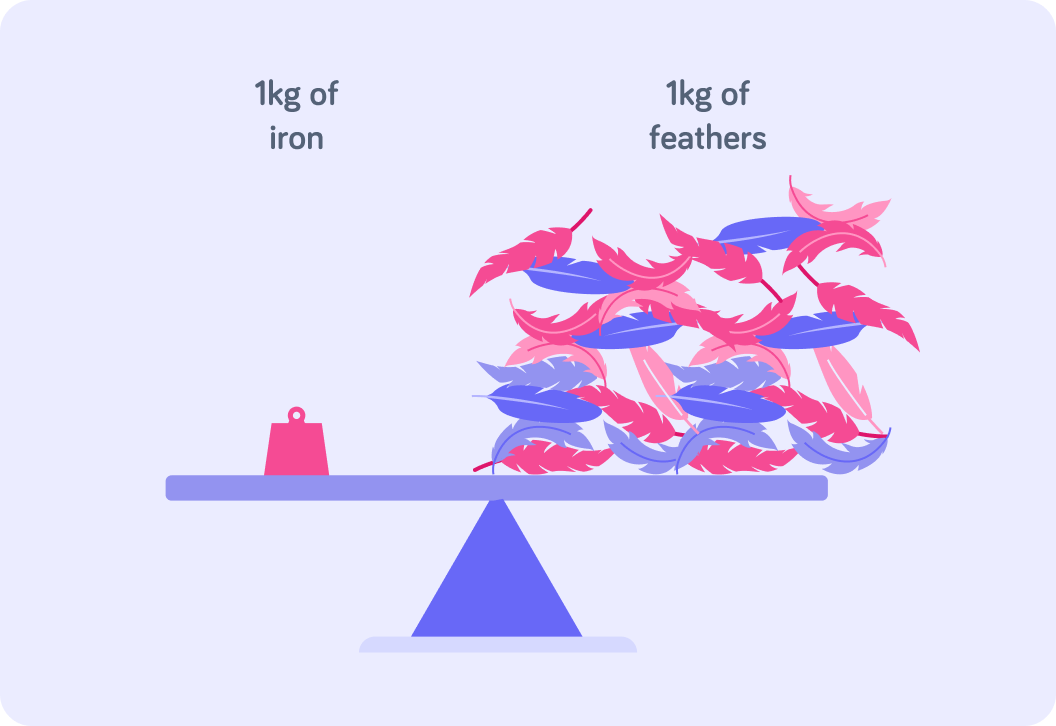
The iron and the feathers also take up the same amount of space*.*


You can say that the iron is more "dense" than the feathers
There is the same amount of mass packed into much smaller space in the iron compared to the feathers.

So an object's density is dependent on it's mass and how much space it takes up. What is another word for how much space an object takes up?

So an object's density is dependent on its _________ and its _________.

You can select multiple answers
Have a look at these three crates
Which one of these crates seems to have the biggest volume?

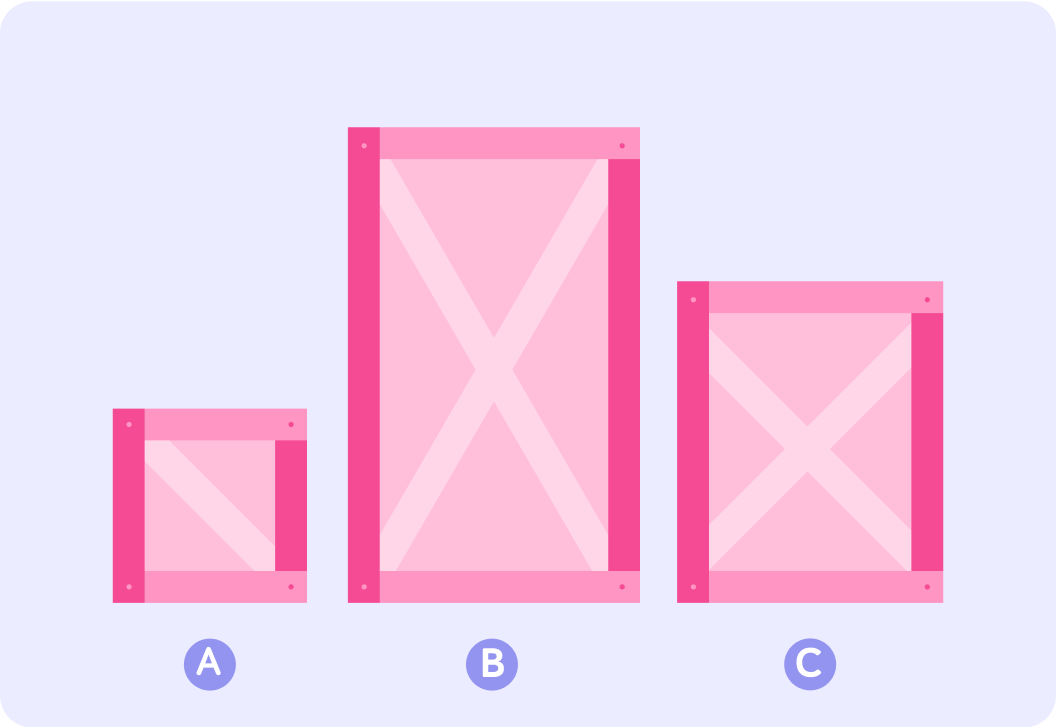
So crate B has the biggest volume. Can you say which crate is the most dense?


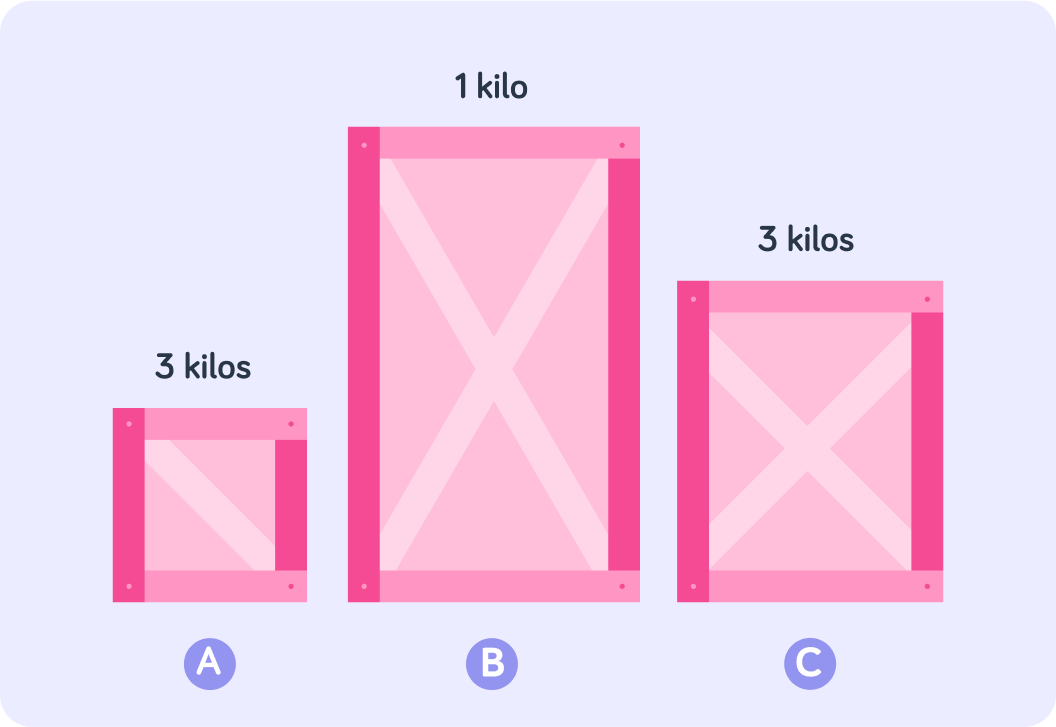
What else do you need to know to find out which crate is the most dense?


Now you also have each crate's mass. Which crate is the most dense?

How dense a substance is depends on how tightly particles are packed in the substance
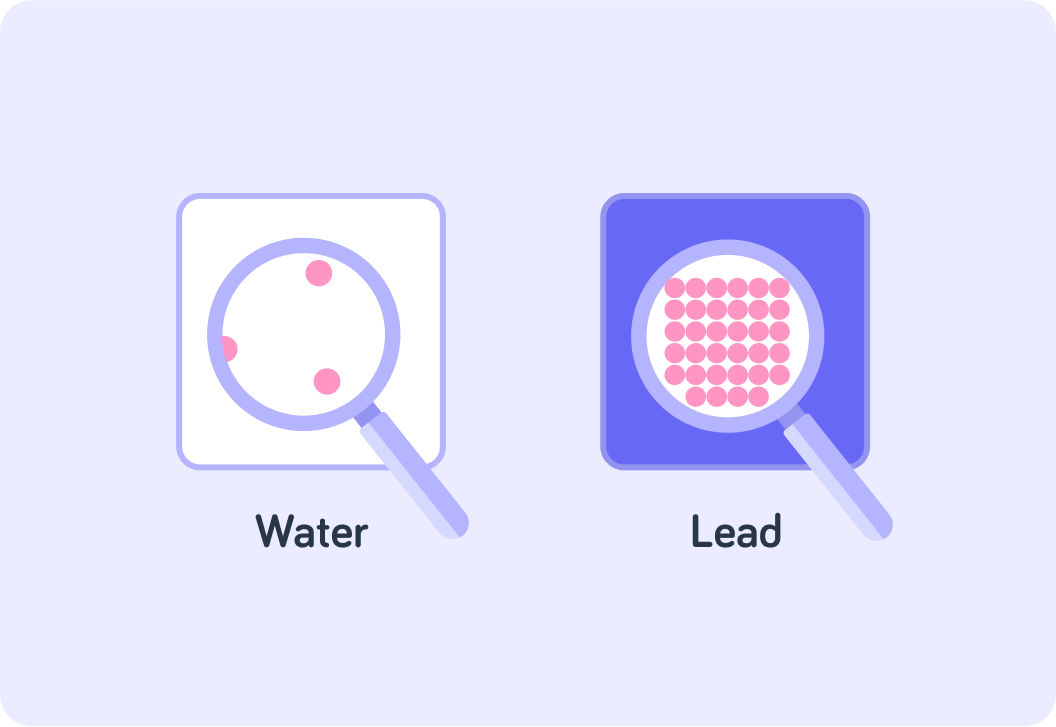
Which one of these substances is more dense?

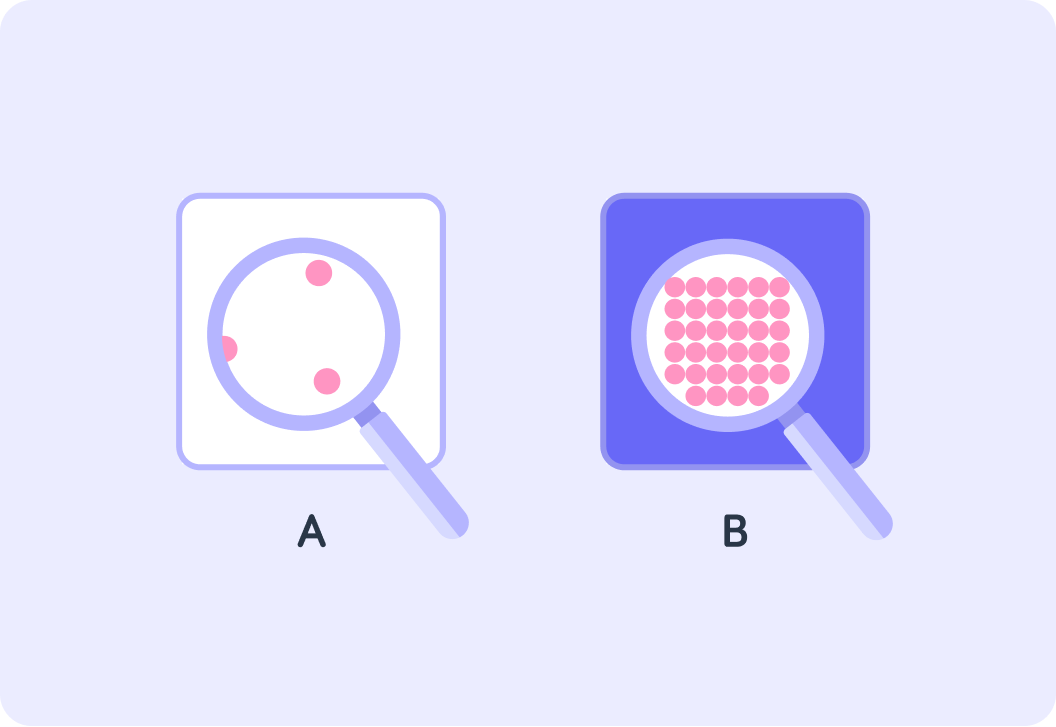
In fact, one substance here is water and the other is lead
Lead is more than 11 times more dense than water!
Magnesium has density 1.74 g/cm3 Copper has density 8.96 g/cm3 Titanium has density 4.51 g/cm3
In which material in the list are the particles most tightly packed?

Which material in the list is the least dense?

Summary!
The density of an object is dependent on its volume and its mass
Volume means how much space the object takes up.

A substance's density is dependent on how tightly packed the particles in that substance are
The more tightly packed, the denser the substance.

For example
Lead is more than 11 times more dense than water!

