YOU ARE LEARNING:
Calculating Change in Pressure/Volume
Calculating Change in Pressure/Volume
P x V = constant
If you increase the volume of a gas, what effect will this have on the number of collisions with the walls of the container?

If you increase the volume of the gas, what will be the effect on the pressure?

As you increase the volume of a gas, the pressure decreases by the same amount due to the decrease in the number of collisions. The value of this is shown as P×V. We say that P and V are inversely proportional. When one goes up, the other goes down by the same amount.
If you increase the volume by a set amount, do you always get the same change in pressure?

If P is pressure and V is volume for a given gas, what can you always say about the value of P×V?

Have a look at this image. What do you notice about the value of P×V, no matter how much you change V and P?
A) Always the same B) Increasing C) Decreasing

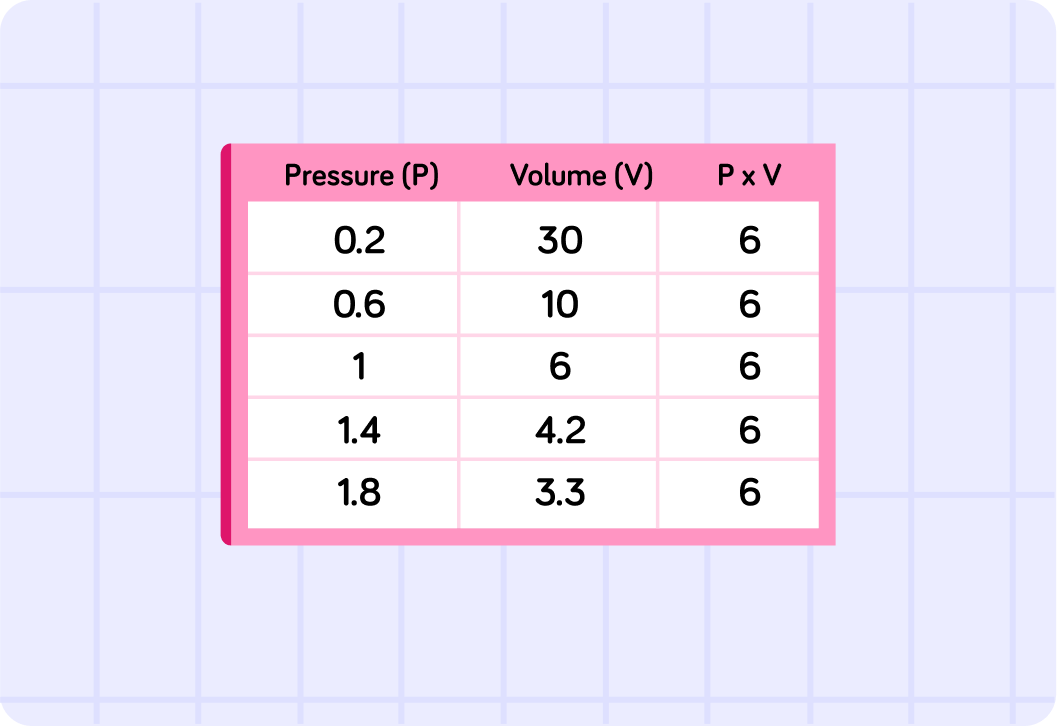
The volume and pressure of a gas are directly related - they are inversely proportional. This means that for any given gas you will always have the same value of P×V, no matter what the volume is or the pressure - as long as the temperature is constant.
If the volume of a gas is 2, and the pressure exerted is 20, then what is the value of P×V?

If a gas has a pressure of 50 when the volume is 4, then what is the value of P×V?

A gas has a pressure of 25 and a volume of 4 giving it a P×V value of 100. If you reduce the volume of the gas what will happen to the value of P×V?

Imagine a gas with a Pvalue of 5 and a V value of 20. If the value of P changed to 10, what would be the new value of V?

A gas has a volume of 5 and a pressure of 4. If the volume of gas is increased to 10, what will the new pressure be?

