YOU ARE LEARNING:
Pressure and Effect on Motion of Molecules
Pressure and Effect on Motion of Molecules
The pressure of a substance is inversely proprtional to its volume.
If you increase the pressure on a gas what happens to the movement of the particles?

Which of the 3 states of matter has the most spaces between the particles?

Which of the 3 states of matter is the **** easiest to compress?

We can compress gasses because there is quite a lot of space between the particles in a gas.
What is the relationship between the volume and the pressure of a gas?

So if we increase the pressure of a gas, how will this affect the volume?

Due to the fact pressure is inversely proportional to the volume of a gas, we can affect the volume by changing the pressure. If we increase the pressure this will compress the gas (make the volume smaller) and vice-versa.
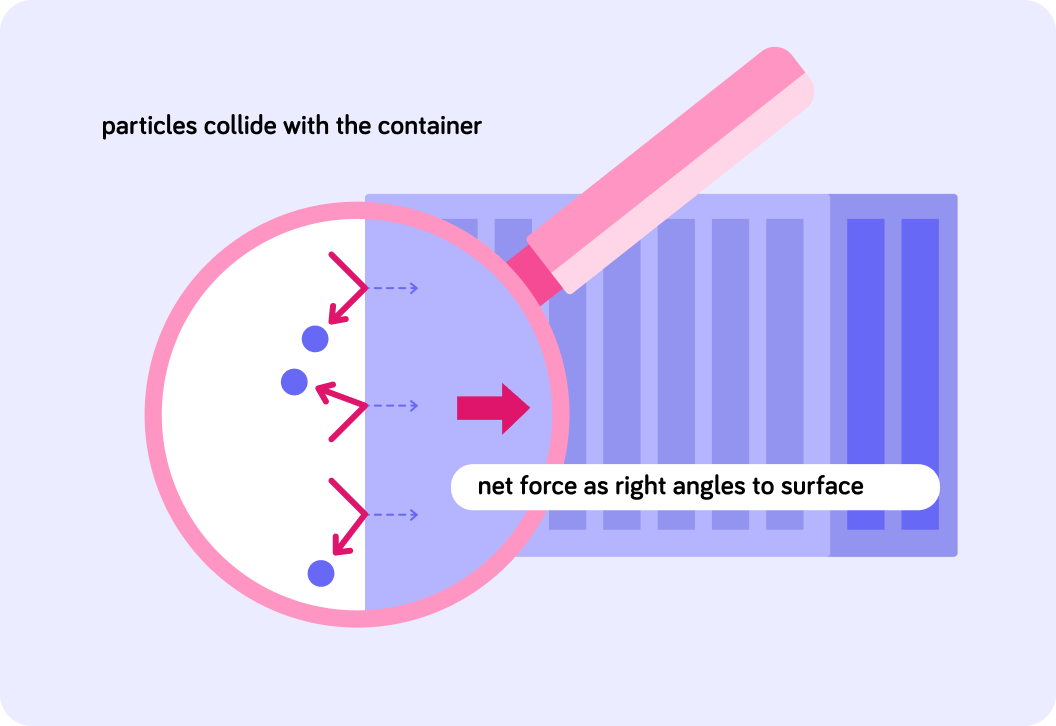
In what direction does the pressure exerted by a gas act on the container?

The direction of the pressure produced by gas particles colliding with the container acts at right angles to the surface of the container.
This image shows the direction of the pressure exerted by the particles colliding with the surface of the container.
You can see it acts at right angles to the surface of the container.
The volume of the container that gas is held in has a massive impact on the pressure the gas exerts!
Does an increase in volume result in more or fewer collisions?

So if there are fewer collisions, will the pressure increase or decrease?

So to sum up, if we change the volume of the gas, this will result in a change in collisions, which will result in a change in pressure.
We can either increase or decrease the volume of the container to either decrease or increase the pressure.
