YOU ARE LEARNING:
Specific Heat Capacity
Specific Heat Capacity
The specific heat capacity of a substance is the amount of energy required to raise the temperature of 1 Kg of a substance by 1 degree Celsius.
True or false? **** All materials of the same state of matter heat up at the same rate?

True or false? The amount of a substance affects how long it takes to heat it up?

Is the amount of energy required to heat an object related to the object's mass and what it is made of?

The amount of energy required to heat an object is related to several factors, including the amount of the substance (mass) and what it is made of.
Now, what do you think the specific heat capacity of an object may be?

Remember! The specific heat capacity of an object is the amount of energy required to raise the temperature of 1 Kg of a substance by 1 degree Celsius.
Which of the following would be an appropriate way of testing the specific heat capacity of a range of different objects?

In order to increase the temperature of a substance we need to give energy to the substance which can be transferred to thermal energy in a way we can measure. Which of these is a method of doing this?

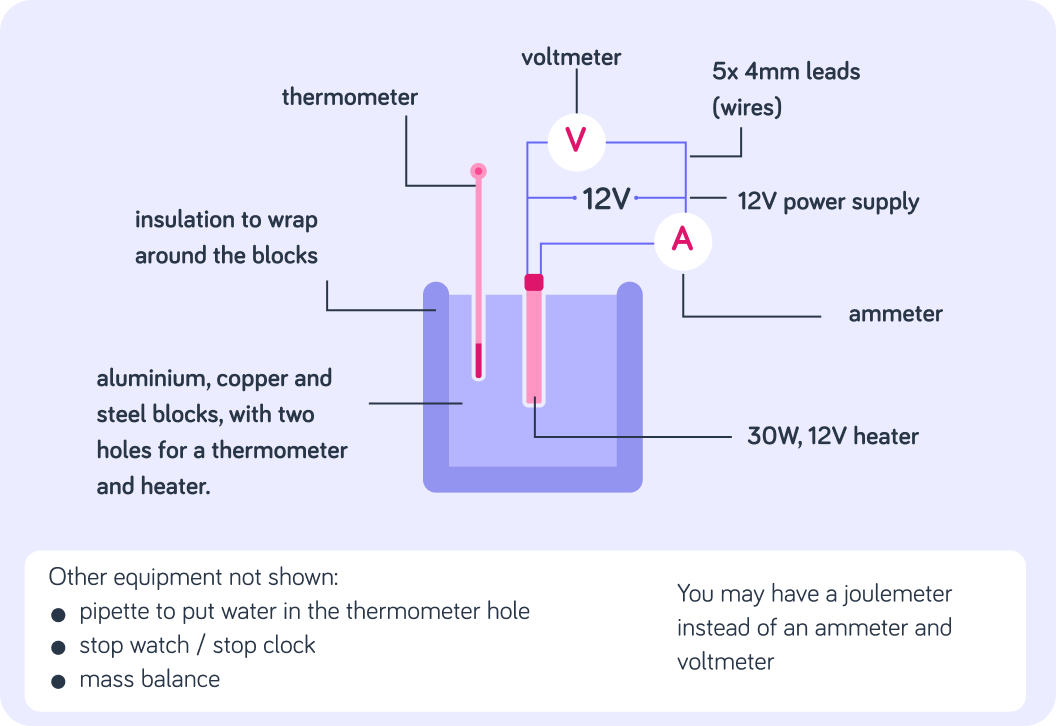
This image shows the practical for specific heat capacity and the method for completing it is as follows.
We have connected a substance to an electrical current and used electricity to increase the thermal energy in the substance. We have kept the voltage the same throughout and measured the current and temperature every minute for 10 minutes.
Which factors affect the thermal energy changes in a system as the temperature increases?Select all correct options from the list below.

You can select multiple answers
Which of these do you think is the correct formula for calculating the thermal energy?

We can calculate the thermal energy change using the formula change in thermal energy=mass×specific heat capacity×temperature change, where the energy is measured in Joules, mass in Kg and temperature change in degrees Celsius.
