YOU ARE LEARNING:
Thermal Expansion
Thermal Expansion
Matter will change state when the particles that make it up gain enough kinetic energy to break the bonds that hold them in place. This KE is obtained through thermal energy, or heating. How much energy is required to change state depends on the substance.
Which of the following are types of energy? Select all the options that you think are correct.

You can select multiple answers
True or false? Energy can be created and destroyed.

If we increase the temperature of a substance, what are we doing to the energy levels of the particles?

The particles in a system have energy in the form of potential energy and kinetic energy. If you increase the temperature of a substance you are putting thermal energy into the system which increases the energy levels of the particles.
What do you think the particles in a substance will use this extra energy to do?

Do the substances in a solid have strong or weak bonds holding them in place?

If particles in a solid have strong bonds between them and the kinetic energy continues to increase what might happen? Select all correct options from the list below.

You can select multiple answers
What do we call it, when the particles in a solid break the bonds holding them together?

True or false? If you heat a liquid, the particles will gain more energy and break the bonds completely and the liquid will become a gas.

Which of the following are changes of state caused by heating a **** substance? Select all the correct answers from the list below?

You can select multiple answers
So if you increase the heat energy in a substance, the particles will use this as kinetic energy and will move faster. If the energy increase is great enough, the particles will break the bonds and change the state of the matter.
What effect will cooling a substance have? Pick all the options that you think are correct.

You can select multiple answers
Which of the following are changes of state caused by cooling a substance? Select all the correct answers from the list below?

You can select multiple answers
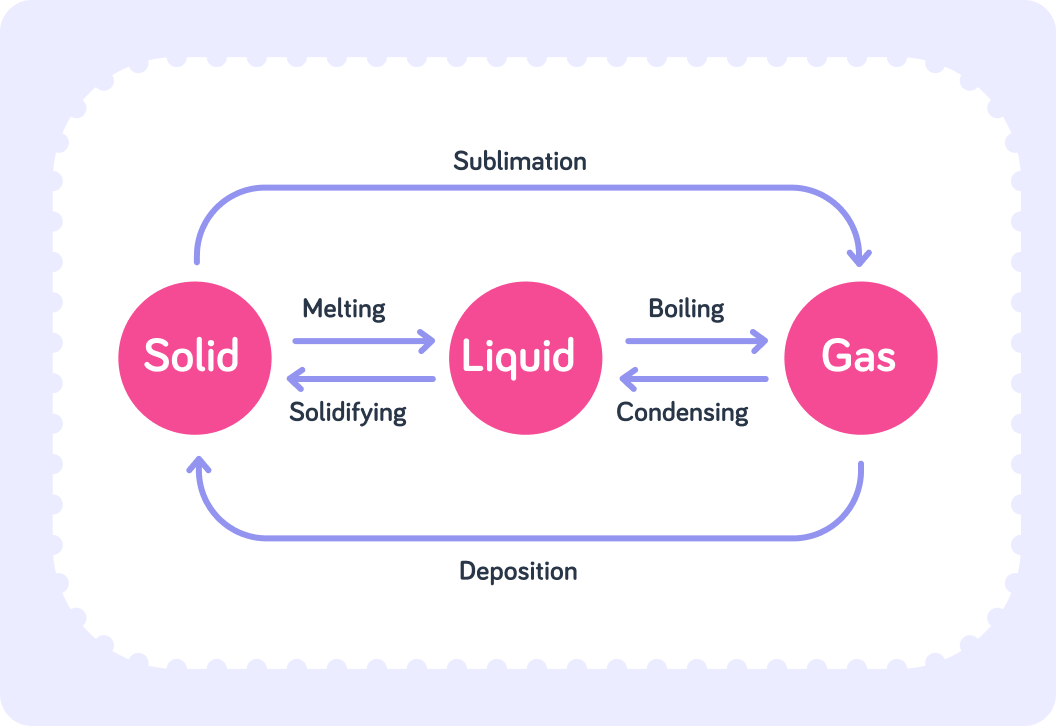
This image shows the different changes of state caused by heating and cooling a substance.
See the above image.
If we have a small cup of water and a large cup of water and heat them both up, which one will heat up quicker?

True or false? The substance an object is made of can affect how quickly the object heats up.

The temperature increase of an object depends on several factors including the substance and mass.
Substance
Different substances have different structures which means they require different amounts of thermal energy to increase the temperature.
Mass
The more mass an object has the more thermal energy it requires to increase the temperature of the substances because there are more particles to excite to increase the kinetic energy.
