YOU ARE LEARNING:
The Rate of Diffusion
The Rate of Diffusion
Different factors affect how quickly diffusion occurs, calculated in an equation (Fick's law).
Now that we know what diffusion is, let's take a look at what determines the rate of diffusion.
What do we actually mean when we say rate of diffusion?

To understand what factors impact the rate of diffusion, let's first imagine that we have a classroom full of students...
If those students had to leave the room, would they leave faster or slower through a big door than through a small door?

If the students were to leave the room, would they leave faster or slower if there were lots of people in the hallway compared to if the hallway was empty?

If the students had to leave not only the classroom, but the school entirely, would their exit be faster or slower?

So the size of the door, the number of people in the hallway and the distance they would have to cover would impact how quickly students in a classroom would be able to complete their escape.
It is similar factors that impact the rate of diffusion in cells.
Surface area
Look at the difference between A and B1. In B1, the border between the two compartments is bigger, which means there is a larger surface area. We can think of this as a bigger door that allows diffusion to happen faster.
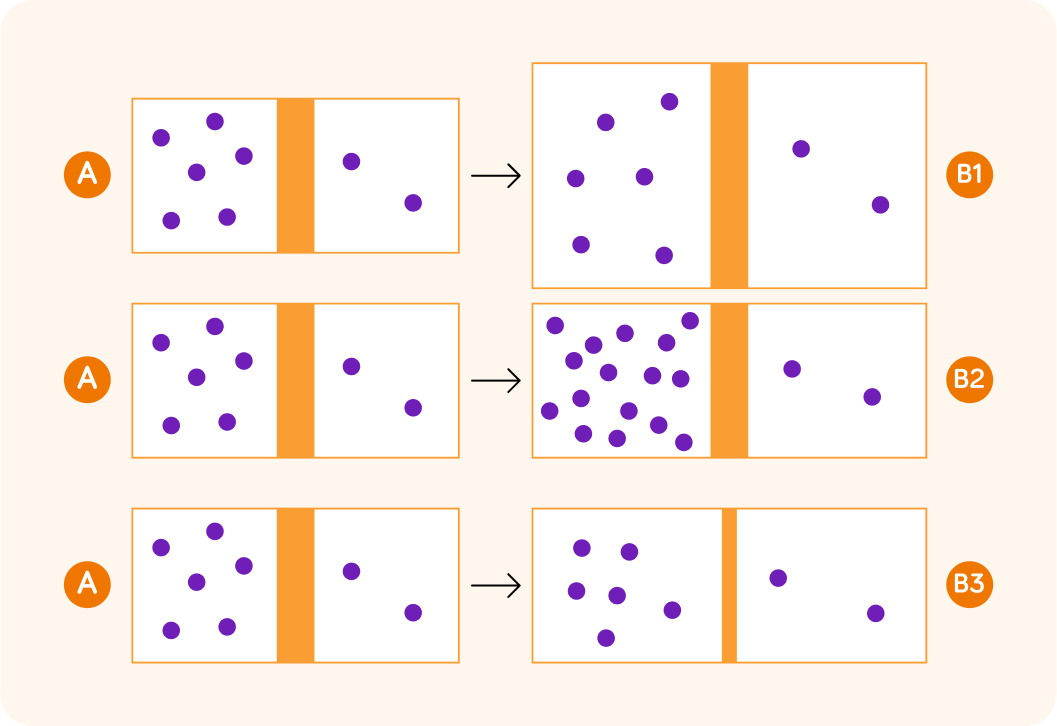
Difference in concentration
Here, look at the difference between A and B2. There is a bigger difference in concentration between the two compartments in B2. This means that the rate of diffusion will be faster in B2 than in A.

Distance
Finally, look at A and B3. The particles in B3 have to travel a shorter distance to get from the department on the left to the department on the right compared to A, so the rate of diffusion in B3 will be higher than in A.

Which factors influence the rate of diffusion?

You can select multiple answers
There is a formula for the rate of diffusion, called Fick's law. Which of these do you think is Fick's law?

The rate of diffusion is determined by three factors: Surface area, difference in concentration and diffusion distance.
These factors combine into Fick's law: rate of diffusion=thickness membranesurface area×concentration difference
If the membrane is twice as thick, what would happen to the rate of the diffusion?

If the surface area becomes 10 times smaller, what would happen to the rate of diffusion?

If the concentration difference is three times bigger, what would happen to the rate of diffusion?

We know that the concentration difference is 5, the thickness of the membrane is 1, and the surface area is 3. What is the rate of diffusion?

We know that the concentration on the left is 3 and on the right it is 4. The thickness of the membrane is 5, and the surface area is 10. What is the rate of diffusion?

If we increase the surface area or concentration difference, we increase the rate of diffusion. If we increase the distance of diffusion, we decrease the rate of diffusion.
This is also seen in Fick's law, where we multiply surface area and concentration difference, and divide with the distance of diffusion.
