YOU ARE LEARNING:
The Rate of Enzyme Activity

The Rate of Enzyme Activity
The rate of enzyme activity depends on certain factors and can be calculated.
First of all, what does an enzyme do to the speed of a chemical reaction?
A) Increases it B) Decreases it C) Nothing

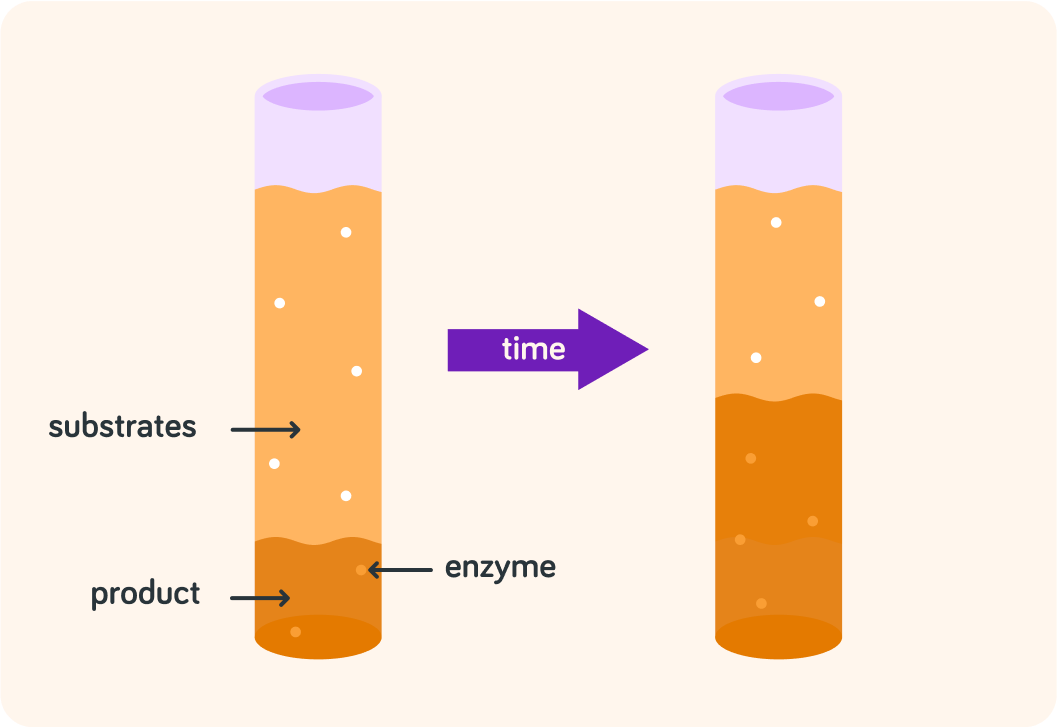
True or false? During a chemical reaction, the amount of substrate increases.


True or false? During a chemical reaction, the amount of product increases.


True or false? During a chemical reaction, enzymes remain unchanged.


We can summarise chemical reactions like this
substrates+enzyme→product+enzyme

This shows a chemical reaction
Substrates turns into product over time
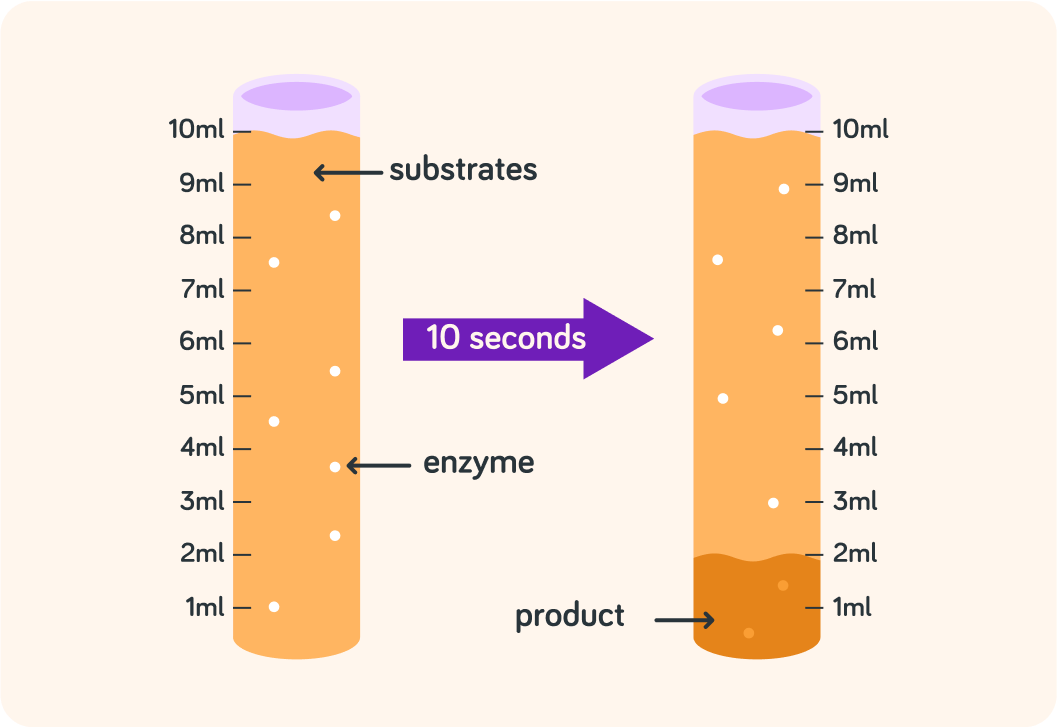
How much product is produced after 10 seconds?


So in 10 s, there is 2 ml of product produced. How much is produced per second then?


The rate of a reaction is how much product is produced in a certain time
For example, here the rate is 0.2 ml/s

This graph shows what happened in a chemical reaction
The more time went by, the more product had been produced.
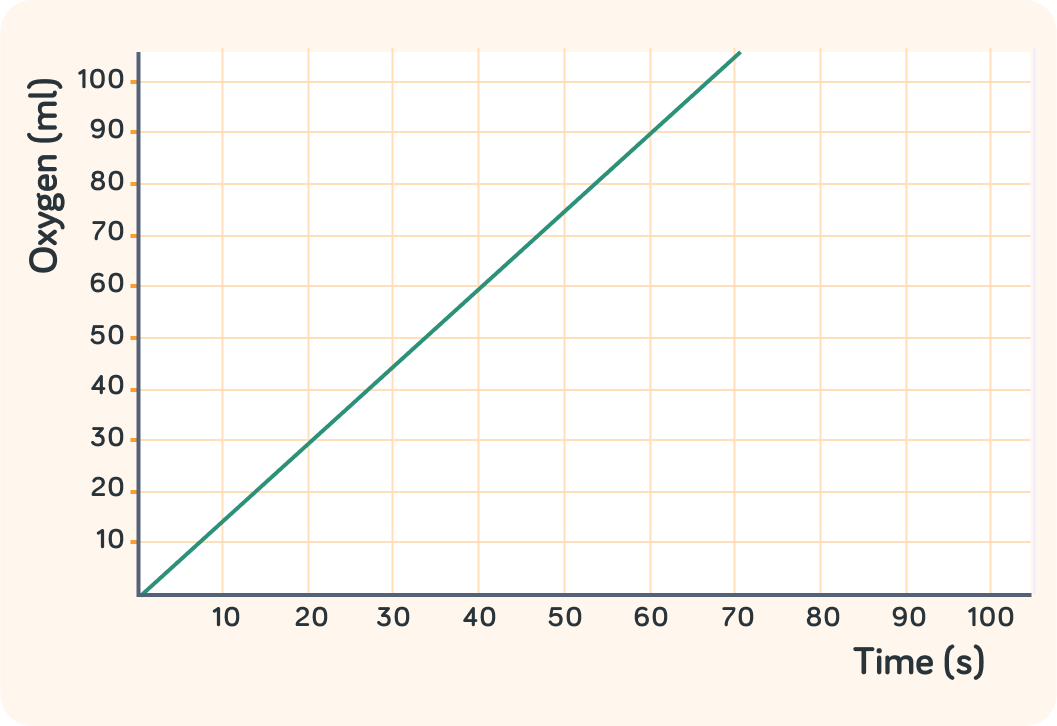
What is the product in this reaction?


So this reaction produces oxygen. What might the reaction be?
A) Digestion B) Respiration C) Photosynthesis


How many millilitres of oxygen was produced between 20 s and 60 s?

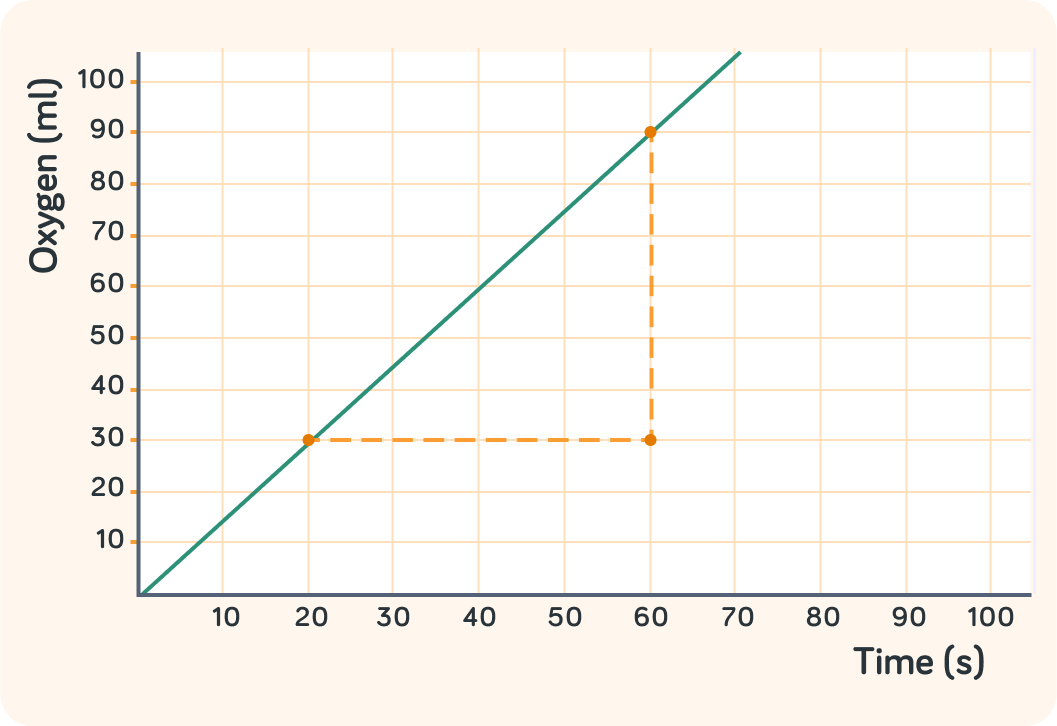
How many seconds passed between 20 s and 60 s?


So in 40 s, 60 ml of oxygen was produced. How many millilitres was that per second?


So you can work out the rate of a chemical reaction by picking two point on a graph that are easy to read
In this example, you worked out the rate of oxygen produced to be 1.5 ml/s

You worked it out like this 40 s90 ml−30 ml=1.5 ml/s. What is the formula for working out the rate of a chemical reaction?


To recap! You work out the rate of a chemical reaction like this
rate of reaction=timechange in amount of product

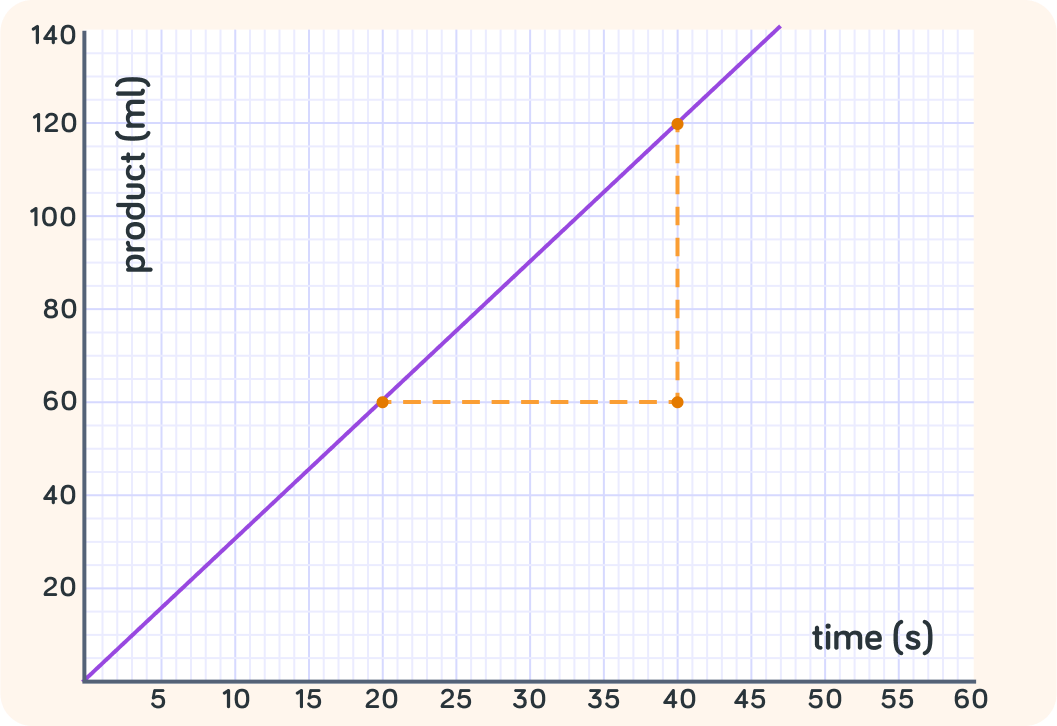
Use rate of reaction=timechange in amount of product to work out the rate of this reaction in ml/s

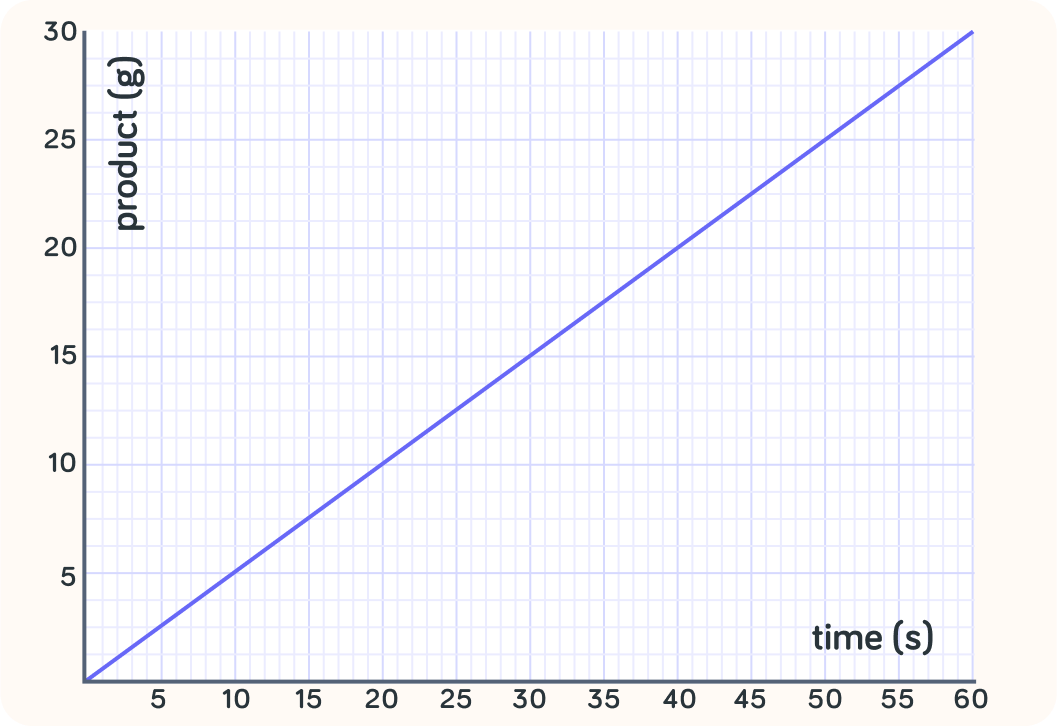
What is the rate of reaction in this example in g/s?

Amount of product isn't always measured in volume, like ml. Pick all the quantities you think you can measure amount of product in.

You can select multiple answers
Which line shows the fastest rate of reaction?
A) The red line B) The purple line C) The green line

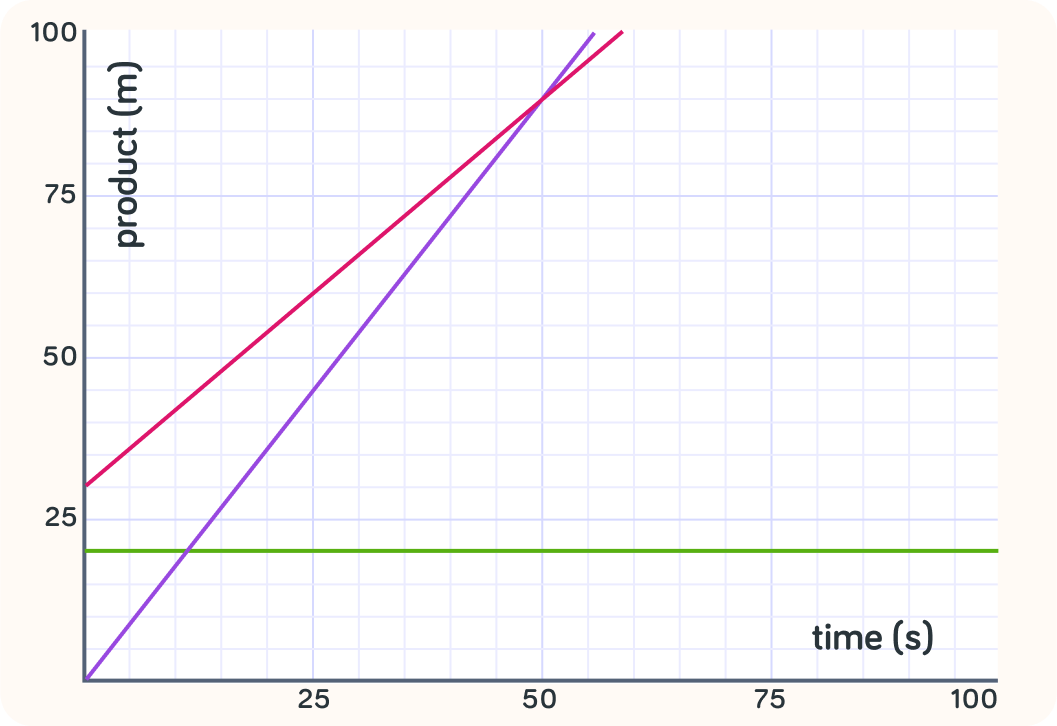
What is the rate of reaction for the green line?


So you can see which reaction is faster by looking at how steep the graph is
The steeper the graph, the faster the reaction. If the graph is flat, the rate is zero.

What is along the x-axis in this graph?

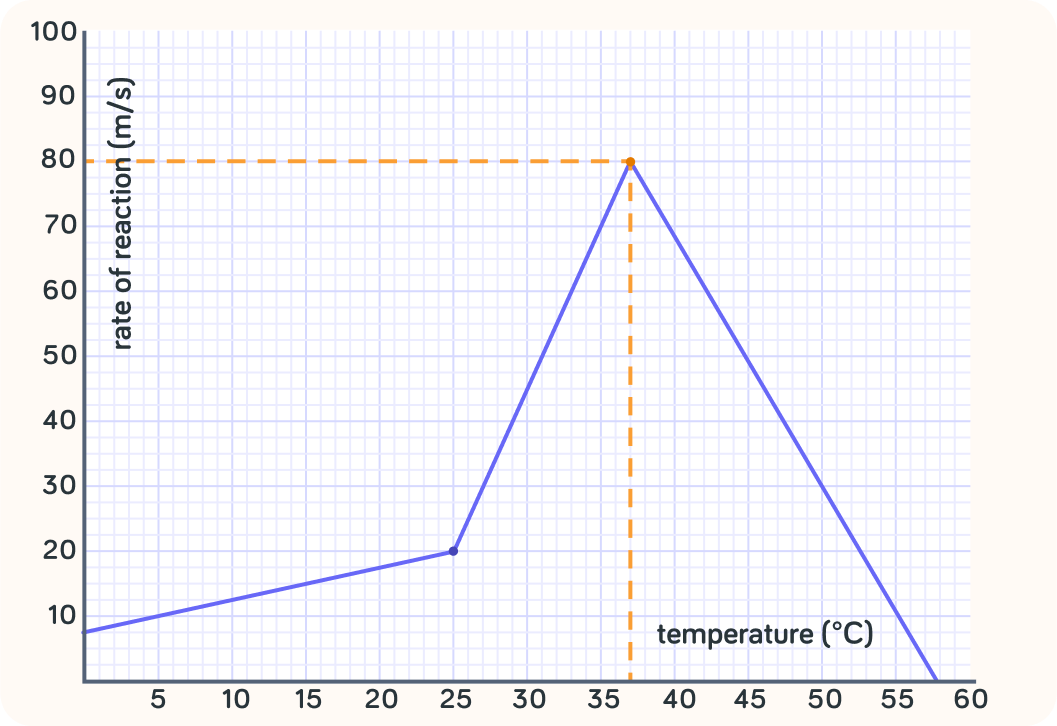
What's up the y-axis in this graph?


True or false? Temperature affects the rate of reaction in this example.


So this graph shows that at certain temperatures, the reaction is faster than at other temperatures
In other words, temperature can affect the rate of reaction.

At which temperature is the rate the highest?


At what temperature is the rate zero?


So the rate of this reaction depends on the temperature
It is fastest at 37 degrees Celsius and zero at 58 degrees Celsius.

The amount of product is not the same as the rate of the reaction.
Does this statement talk about the amount of product or the rate of the reaction? A chemical reaction produced 24 ml of product.

Does this statement talk about the amount of product or the rate of the reaction? A chemical reaction produced 0.02 g/s

Amounts
Physical quantities, like mass, volume and moles (grams, millilitres etc.)
Rates
How fast amounts are changing (grams/second, millilitres per second etc.)
Summary! The rate of a chemical reaction
How fast the chemical reaction changes substrates into product, for example 0.02 g/s

You calculate the rate of reaction like this
rate of reaction=timechange in amount of product

You can tell by the steepness of a graph how fast the reaction is
The steeper the graph, the faster the reaction. A flat graph means the reaction is zero.

The rate of a reaction can depend on for example temperature
This reaction is fastest at 37 degrees Celsius and zero at 58 degrees Celsius.

