YOU ARE LEARNING:
Activity

Activity
The activity of a radioactive sample is the overall rate of decay of all of its unstable isotopes.
This lesson looks at the concept of activity. But first, what is it that makes a substance radioactive?

A radioactive substance contains unstable isotopes. These are nuclei of the same element but with a different number of neutrons, which makes them unstable. In order to become stable, these nuclei emit radiation in the process of radioactive decay.
Can you predict when a single unstable isotope will decay?

Radioactive decay is a completely random process. This means that we can't know when a particular unstable isotope will decay. However, if we have a big enough sample of a radioactive substance, we can still find out two very useful things. We can find out its activity and its half life.
What do you think we mean when we talk about the activity of a sample of radioactive substance?

The activity of a radioactive sample is the average number of isotopes decaying per second, or the overall rate of decay of **** all unstable isotopes in the sample. Activity is measured in Becquerels (Bq).
What do you think a Becquerel (Bq) might be equal to?

If a sample of radioactive substance has an activity of 600 Bq, then how many isotopes decay per second in that sample? Remember that 1 Bq=1 decay per second.

So if a sample of radioactive substance has activity 600 Bq, that means that 600 isotopes decay per second in that sample. Which one is the correct formula for working out activity?

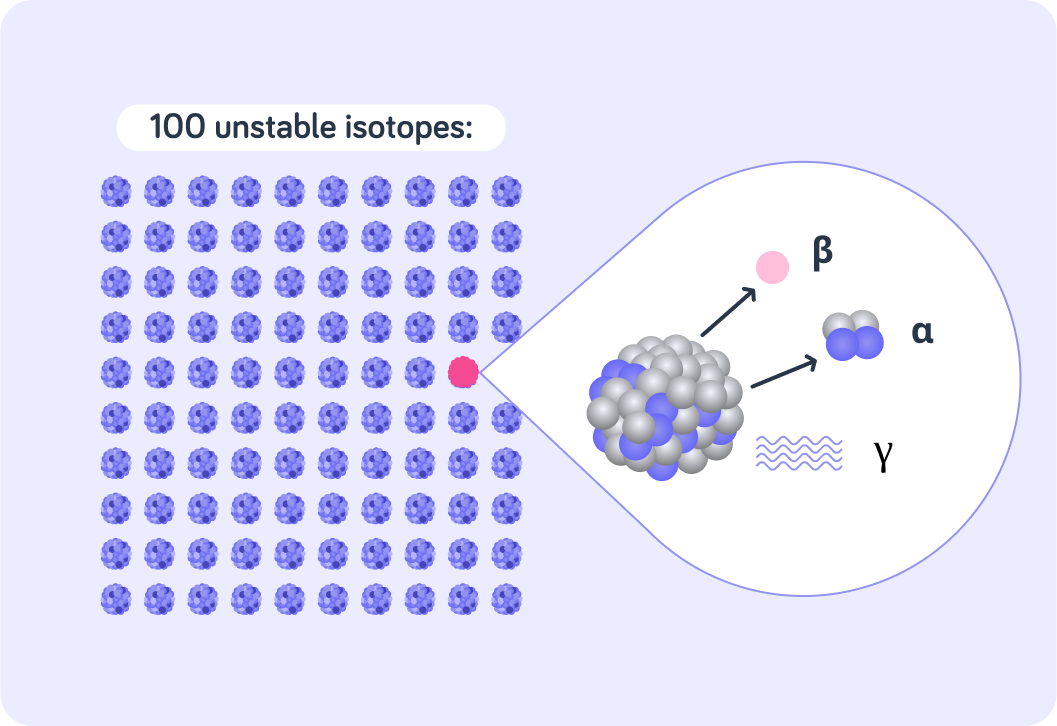
This is a radioactive sample with 100 unstable isotopes.
Each of these isotopes can decay by emitting radiation in order to become stable. The decay process is completely random, so we can't know when any particular particle will decay.
As time goes on, what happens to the number of remaining unstable isotopes? Does it increase or decrease?

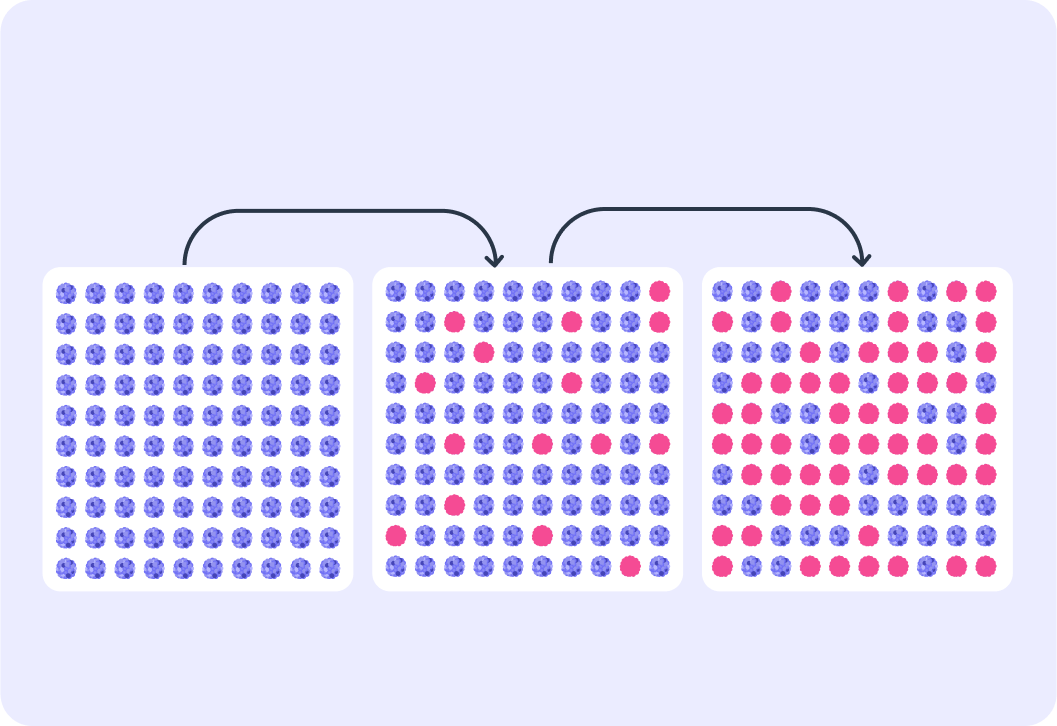
So when a sample of radioactive substance decays, the number of unstable nuclei decreases.
This means that the rate of decay of all the isotopes (the activity) also decreases over time.

We can define the half-life of a sample of radioactive substance. What do you think it means?
A) The time it takes for the number of unstable isotopes to halve. B) The time it takes for the amount of substance to halve. C) The time it takes for the effects of radiation to halve.


So half-life is the same as the time it takes for the activity of the sample to halve.
More on that in later lessons.

