YOU ARE LEARNING:
Calculating Pressure

Calculating Pressure
Changing the volume or amount of gas in a container will affect the pressure within it. We will also think about the kind of relationship they have to each other, whether it increases or decreases.
If 30 blindfolded people were moving around randomly in a room and you then increased the size of the room, would they collide more with the walls or less with the walls?

True or false? if you decrease the size of a container, but keep the same amount of gas within this container, the particles in the gas will collide less with the walls of the container.

If you decrease the size of the container that holds a fixed amount of gas, what will happen to the pressure the gas exerts?

So the pressure exerted by a gas is directly related to the size of the container the gas is held in - also known as the volume of gas. If you increase the volume there is more space for the particles to move around in and therefore fewer collisions, which means lower pressure.
If 30 blindfolded people were moving around randomly in a room, and you introduced another 30 blindfolded people, would there be more or fewer collisions with the walls?

True or false? If you **** increase the amount of gas in a container then there will be more collisions between the gas particles and the walls of the container.

If you increase the amount of gas in a container, what effect will this have on the pressure exerted by the gas?

To sum up, if you increase the amount of gas in a container you are increasing the amount of particles. This means less space for them to move around in which means more collisions with the walls of the container. More collisions with the walls of the container means the gas exerts a greater pressure.
If there are more collisions, is the rate at which particles collide increasing or decreasing?

True or false? Volume of gas is proportional to the pressure exerted by the gas.

The amount of pressure exerted by a gas, known as P, is inversely proportional to the volume of gas in a container, known as V. So as Vincreases, P decreases by the same amount for each change of V.
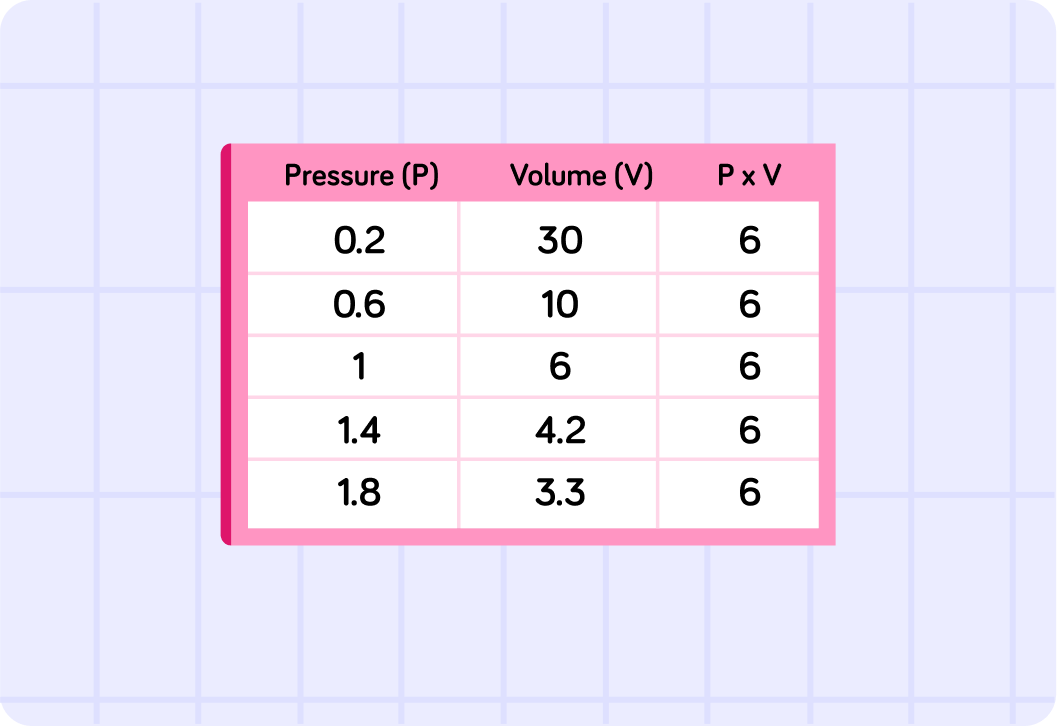
If P and V are inversely proportional, what can we say about the result of p×v for a range of different volumes of gas and pressures?

We are going to look at a range of pressure and volume situations, for each one you need to calculate P×V
Volume of gas 50 exerts a pressure of 1.2, what is the result of P×V?

The volume of the gas decreases to 37.5 and the pressure of gas increases to 1.6 what is the product ofP×V?

The volume of the gas is decreased again to 30 which causes the pressure to increase to 2. What is the value ofP×V?

This image shows that becausePandVare related you will always get the same answer in any given situation for P×V. If you look at the table you can see that for every change in volumeVthe pressurePchanges a uniform amount which results in the result ofP×Valways being the same.
This is known as Boyle's Law.