YOU ARE LEARNING:
Pressure in Terms of the Motion of Particles

Pressure in Terms of the Motion of Particles
In this lesson you will learn what pressure means in the context of small particles and how this can be defined in terms of force, area and direction.
Why do you think you need to pump air into the tyres of a car? Why doesn't the air just stay in the tyres forever?

Which of the following states of matter has the largest movement of particles?

Which of these best describes the movement of particles in a gas?

To recap, the particles in a gas have **** no forces of attraction (bonds) between them. This means that the particles are **** free to move and are able to move in random directions and at random speeds.
True or false? The particles in a gas will collide with each other as they move around the container.

True or false? The particles in a gas will exert a force on the sides of a container through colliding into them as they move around.

When the particles collide with the side of a container they exert a force on this container, the force per unit area is known as the pressure.
When the particles in a gas collide with the sides inside a container, in which direction do the gas particles then travel?

So when the particles of a gas collide with the inside of a container, they exert a force. In which direction do you think this force acts?

The direction of the force in relation to the container is at ______ ________?

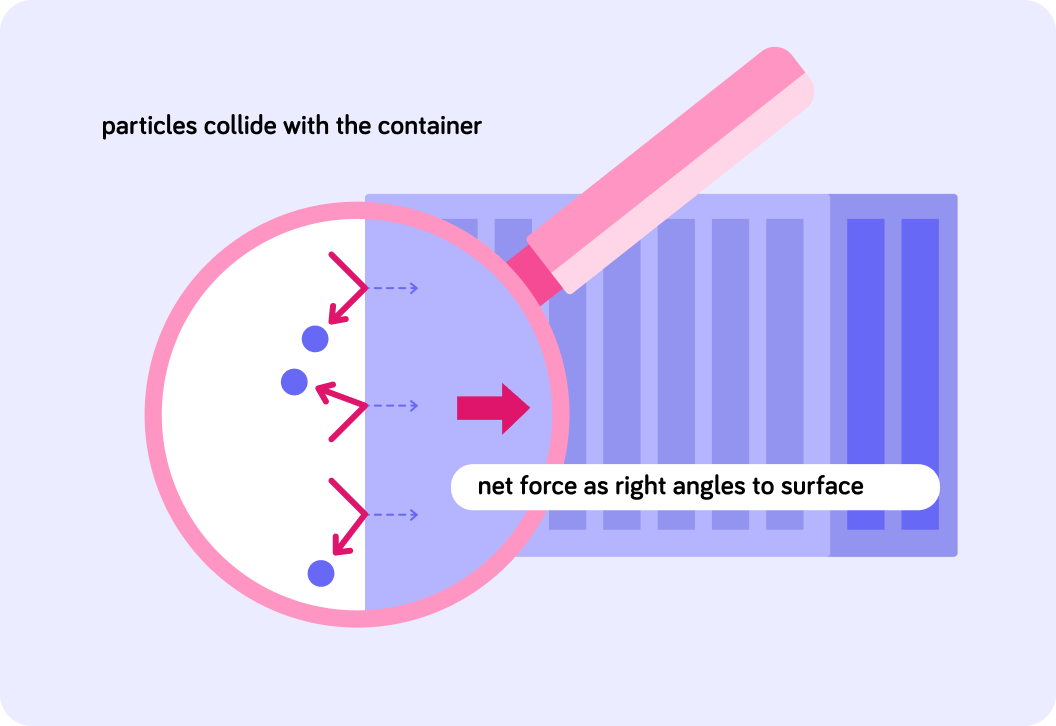
To sum up, the force exerted by the particles colliding with the side of the containers acts at right angles to the container.
The image shows the force exerted, compared to the particles colliding with the container.

