YOU ARE LEARNING:
Electrons and Their Orbits
Electrons and Their Orbits
Electrons orbit atoms in shells, and can be removed or added to created ions.
The table shows the charge and relative mass of subatomic particles, but the values for an electron are missing! An electron has a negative charge, so what value do you think is missing for the charge of an electron?

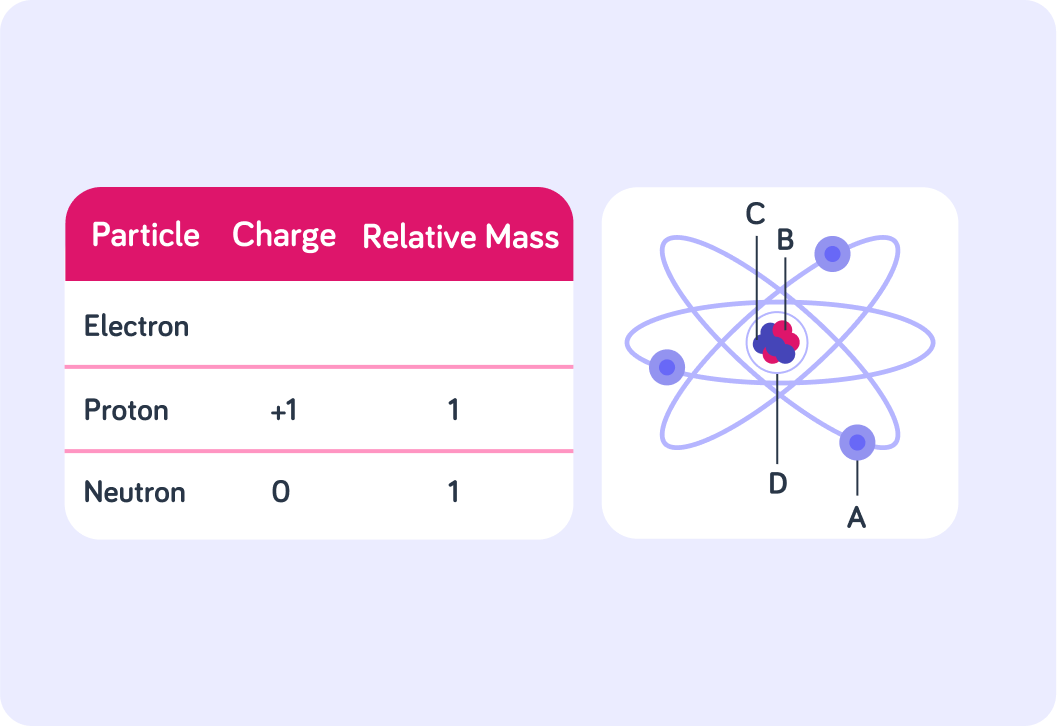
The mass of an electron is so small that it is insignificant to the overall mass of the atom, so what value do you think is missing from the table for the relative mass of an electron?


Now look at the image of an atom. Which arrow is pointing to the nucleus? Answer A, B, C or D.


Which arrow is pointing towards and electron? Answer A, B, C, or D.


What number in the periodic table do you use to find out how many electrons an atom has?

Electrons are the subatomic particles which orbit the nucleus of an atom. They are negatively charged, and they are much smaller and have much less mass than the nucleus. A neutral atom has the same number of protons and neutrons, so the atomic number can tell you how many electrons an atom has.
How electrons are arranged in an atom
Electrons are arranged in shells around the nucleus.
Each shell refers to an orbital for electrons which have a certain energy. This is why shells are also called energy levels.
Where do you think the shell with the lowest energy level lies?

The nucleus of an atom is surrounded by electrons. These are arranged in energy levels at different distances from the nucleus, where the lowest energy level is closest to the nucleus.
Electrons usually occupy the lowest energy levels available to them.
The arrangement of electrons differs from element to element, and is responsible for the chemical properties of each element. Depending on how many electrons an atom has, they will have a different number of shells. What do you think we call the highest energy level an atom has?

A sodium atom has 2 electrons in first shell, 8 in second shell and 1 in third shell. We call the arrangement of electrons the electronic configuration of the atom. So in this case it is 2.8.1 How many electrons does the atom have in total?

A sodium atom has electron configuration 2.8.1. A potassium atom has configuration 2.8.8.1. How many electrons can the first shell of an atom hold?

The maximum number of electrons in each shell, going from the middle to the outside, is 2, 8, 8, 18. So if a sodium atom has a configuration of 2.8.1, is its third and outer shell full?

When the outer shell has the maximum number of electrons, the electron shells are said to be full. The inner shells of an atom are always full.
An atom that has the maximum number of electrons in its outer shell will be stable. This means that it will not react with other atoms.
Atoms tend to want to have a full outer shell of electrons.
That way they are more stable.
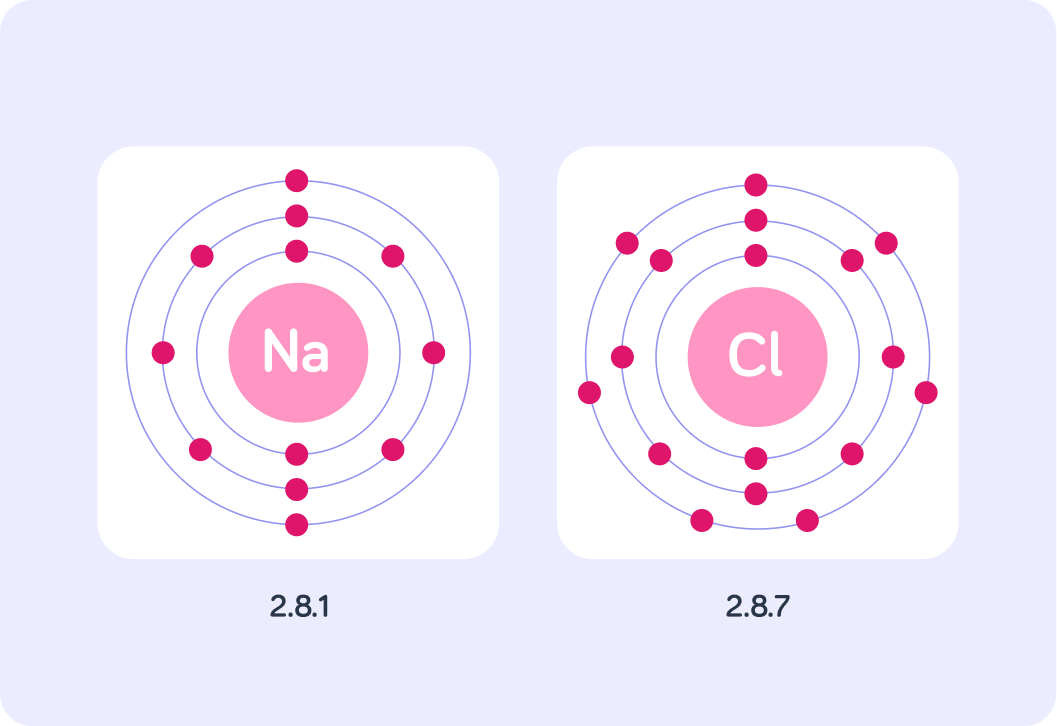
Look at the electron configurations of sodium and chlorine. If sodium were to achieve a full outer shell, how many electrons would it have to gain?


Gaining 7 electrons is really difficult!
Instead, sodium could lose its 1 electron in the third shell. That way its outer shell would become its full second shell.

How many electrons would chlorine need to gain to have a full outer shell?


So sodium wants to lose an electron, and chlorine wants to gain one. Handy!
A) Sodium and chlorine are not reactive together. B) Sodium and chlorine are reactive together. C) Sodium and chlorine have similar properties.


This explains why sodium and chlorine easily bond to form sodium chloride, NaCl.
They just have to transfer just one electron. We call this ionic bonding.

Atoms are most stable when their outer shell is full, and are most reactive when it is not. This explains why the noble gases in group 8 of the periodic table are very unreactive. It is also why group 1 and group 7 elements bond well with each other.
If an atom of sodium were to transfer an electron to an atom of chlorine, what charge would each atom have? Pick the correct one for sodium and the correct one for chlorine.

You can select multiple answers
When atoms lose or gain electrons in the process of creating a full outer shell, the overall charge of the atom is affected. If electrons are lost, the atom will become a positively charged ion, whereas if electrons are gained the atom becomes a negatively charged ion. This is what is called ionisation.
Exciting electrons
An atom can become ionised when an electron leaves its outer shell. In order to do this, the electron must...

When an electron absorbs energy, it jumps up to a higher energy level. We say that it has become excited.
If the electron gains enough energy, it can leave the atom altogether and the atom becomes a positively charged ion.
Most often however, electrons don't gain enough energy to leave the atom. Instead they jump to a higher energy level. After some time however, they will want to go back to the lower energy level to be more stable. In this case they will...

The energy the electrons absorb and emit are in the form of electromagnetic radiation.
The difference in energy levels that an electron jumps between corresponds to a particular frequency of the electromagnetic spectrum.
The absorption of radiation can be analysed by an ‘absorption spectrum’. The different black lines show the frequencies __________ by electrons in atoms, allowing them to jump to higher energy levels.

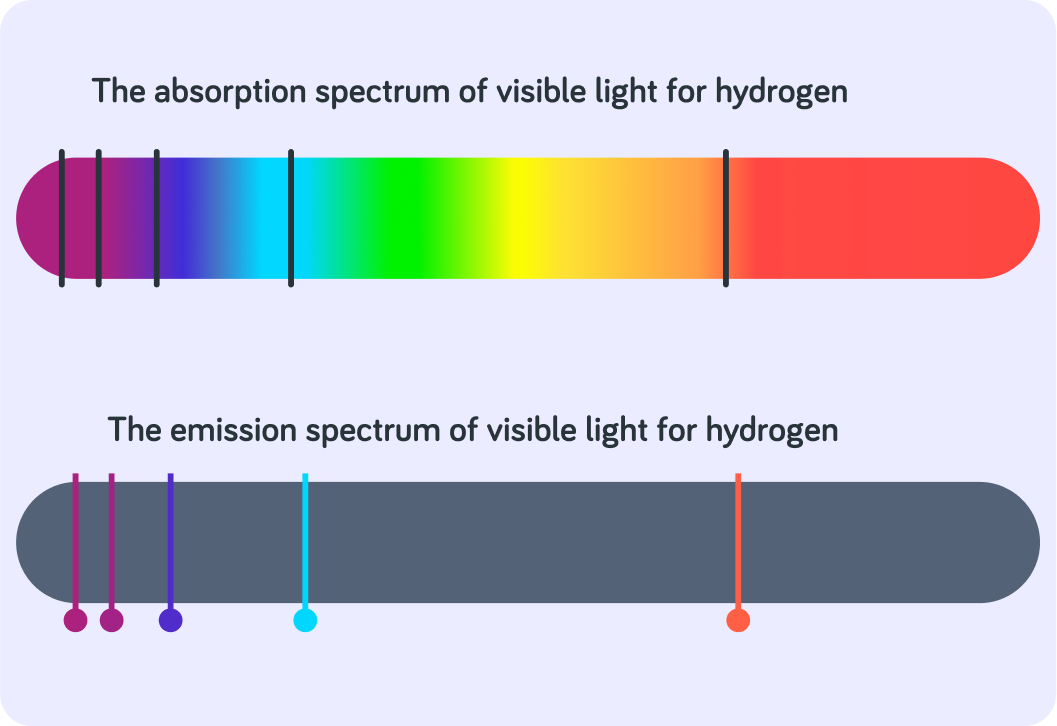
The emission of radiation can be analysed by an ‘emission spectrum’. The different coloured lines show the frequencies __________ by excited electrons falling back down to lower and more stable orbits.


