YOU ARE LEARNING:
What Are Isotopes?
What Are Isotopes?
Isotopes are atoms of the same element (same atomic number) but with different numbers of neutrons (different nucleon number).
Carbon has an atomic number of 6. This means that carbon has...

Atomic Number
The atomic number, also known as the proton number, tells you the number of protons an element has in its nucleus. What other quantity it this equal to?

The atomic number also defines an element.
For example, an atom of carbon will always have 6 protons in its nucleus, so will always have an atomic number of 6.
Both these symbols represent carbon atoms...
but something is different!
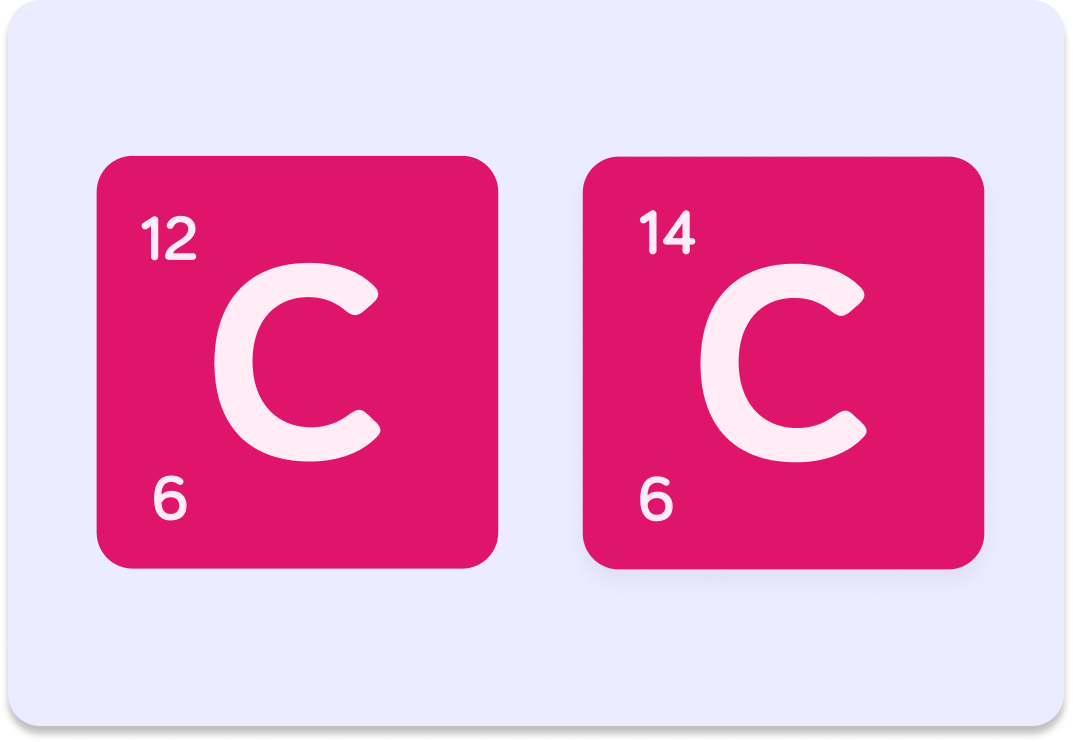
The number 6 is carbon's atomic number, but what do you think the numbers 12 and 14 mean?
A) Atomic mass B) Electron number C) Neutron number
Answer A, B or C.


Atomic mass is how much mass the atom has. It is also the number of...
A) subatomic particles. B) protons and neutrons. C) neutrons and electrons.
Answer A, B or C.


So carbon atoms always have 6 protons, but each of these carbon atoms have different masses. That means they must have different numbers of ________________.


All atoms of a particular element have the same number of protons in their nuclei. However, they don't always have the same mass. When atomic masses differ it is because the atoms have different numbers of neutrons.
We call these isotopes - atoms of the same element, but with different numbers of neutrons in the nucleus.
Pick all the options you think say something true about isotopes.

You can select multiple answers
So far we called the mass of an atom the mass number, or atomic mass. It can also be called the nucleon number. What type of subatomic particle do you think a nucleon is, then?

This image shows the three naturally occurring isotopes of hydrogen.
They all have 1 proton and 1 electron, but they have differing numbers of neutrons.
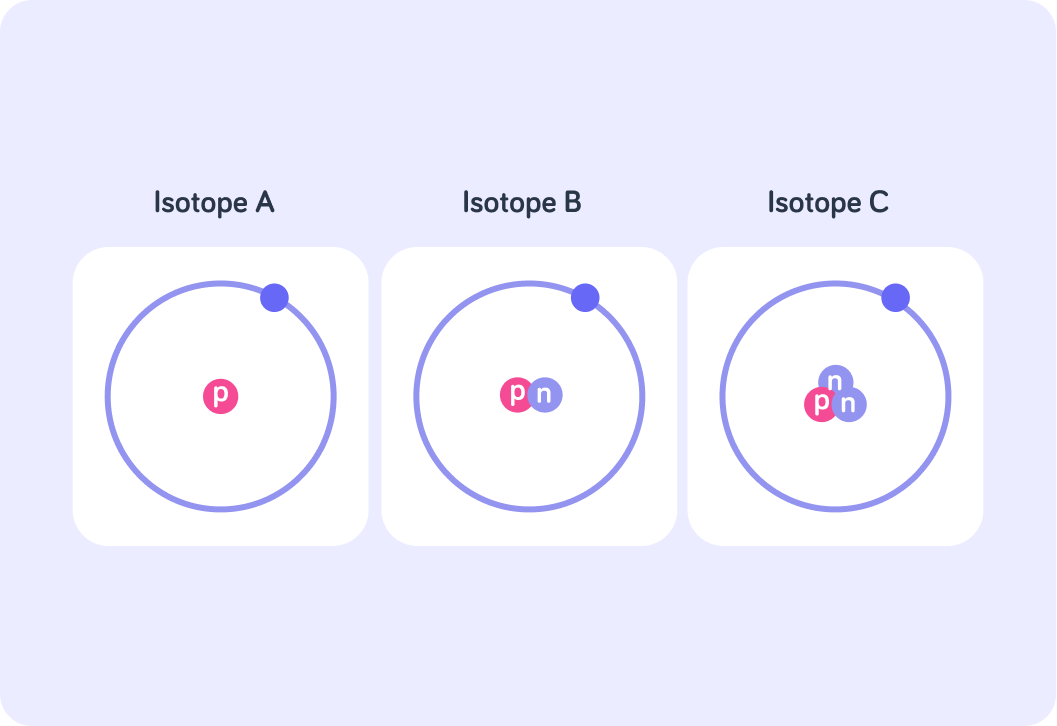
Which isotope has a nucleon number of 3? Answer A, B, or C.


Which isotope has a nucleon number of 1? Answer A, B, or C.


If the bottom number is the atomic number, and the top number is the nucleon number, then which isotope do you think is represented by the notation 13H ? Answer A, B, or C.


So isotope C can be represented as 13H, because it has nucleon number 3 and atomic number 1. How would isotope A be represented?
Use the fraction tool like so bottomnumbertopnumber.


Remember that the nucleon number is the number of protons and neutrons an atom has. How many neutrons does carbon-12 have in its nucleus?


How many neutrons does carbon-14 have in its nucleus?


If you look in the periodic table, you will see that the relative atomic mass of chlorine is recorded as 35.5. You can't have half a neutron, so how can this be explained?

How are isotopes used to calculate relative atomic mass?
There are two isotopes of chlorine. 75% of chlorine are isotopes with 35 nucleons, and 25% of chlorine are isotope with 37 nucleons. Let's pretend we have 100 isotopes in total. What would their combined relative atomic mass be?

Now, what is the relative atomic mass of a single chlorine atom? Remember it is an average.

So to find the relative atomic mass...
You find the sum of the mass of individual isotopes, multiplied by their abundances, and divide by the total number of isotopes. For example 100(75×35)+(25×37)=35.5
Nearly all of the elements in the periodic table have isotopes. However, for most of them the vast majority of atoms are one particular isotope, so the relative atomic mass is close to a whole number.
