YOU ARE LEARNING:
Nuclide Notation
Nuclide Notation
We use nuclide notation to represent an element in terms of its atomic number and nucleon number.
The periodic table arranges all the elements in a particular order. The first element listed is hydrogen (1), then helium (2), lithium (3) and so on. The elements are arranged in the order of which number?

Every element is defined by its atomic number. **** The existence of ions and isotopes shows us that the number of electrons and neutrons are not always the same for every atom of a particular element. However, the atomic number always remains the same.
The atomic number of an element tells you...

You can select multiple answers
We use nuclide notation to represent an element, and its number of subatomic particles.
The image shows what we mean by nuclide notation. It is a general formula, not a specific example.
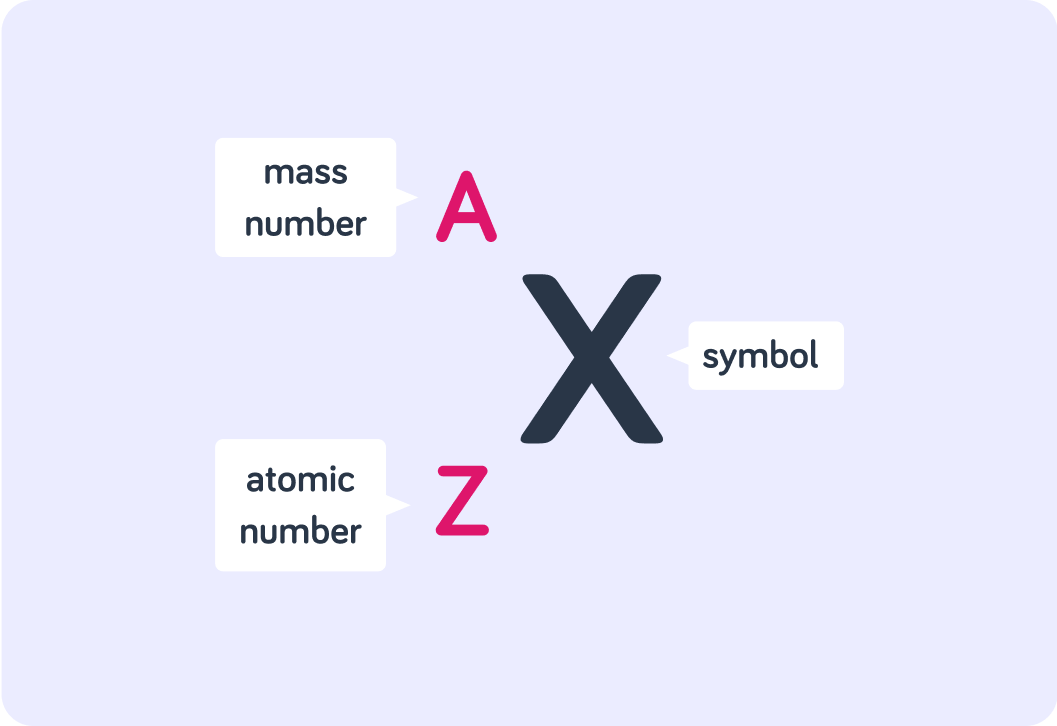
The chemical symbol X is the symbol we use for any element in the periodic table. If this were the nuclide notation for an isotope of carbon, which letter would be used for the symbol X?


You can also find the atomic number (Z) of any element in the periodic table. In the nuclide notation for an isotope of carbon, what number would we fill in instead of Z?


Let's say this is an isotope of carbon that has 7 neutrons. What number would be used for the mass number (A)?


So this is what the nuclide notation looks like for a carbon-13 isotope with 7 neutrons.
The chemical symbol is C, the atomic number (Z) is 6 and the mass number (A) is 13.

A (mass number) is the total number of subatomic particles in the nucleus, and Z (atomic number) is the number of protons in the nucleus. Now, what is the general formula for calculating the number of neutrons in the nucleus?

Nuclide notation is used to represent an element, and its number of subatomic particles. Will every atom of a particular element have the same nuclide notation?

So the mass number is the number of nucleons in the nucleus. Therefore, we also sometimes call it the nucleon number. What do you think a nucleon is?

Look at the nuclide notation for two isotopes of neon. What is the atomic number of neon?

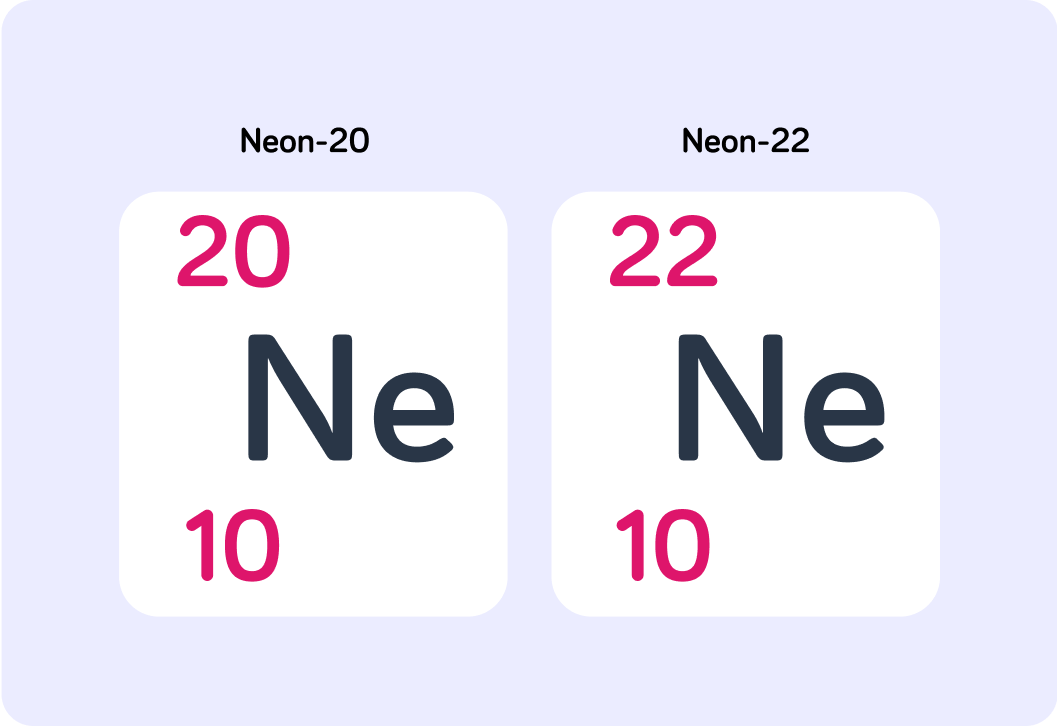
How many neutrons does neon-22 have?


How many neutrons does neon-20 have?


If neither of these atoms is a charged particle, how many electrons do they both have?


An element's characteristics of reactivity and bonding are determined by the number of electrons in its orbit. So which part of the nuclide notation tells us something about the element's reactivity and bonding properties?

How would you write the nuclide notation of hydrogen? Use the fraction tool like so ZAX.

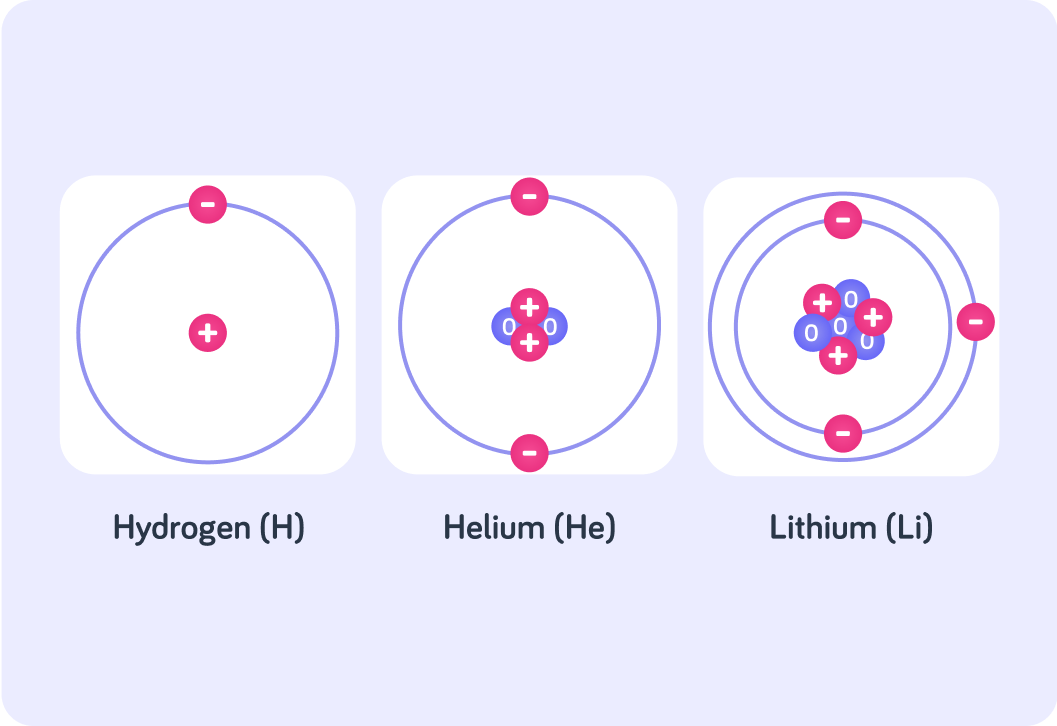
How would you write the nuclide notation of helium? Use the fraction tool like so ZAX.


How would you write the nuclide notation of lithium? Use the fraction tool like so ZAX.


