YOU ARE LEARNING:
How We Got to This Model
How We Got to This Model
From the plum pudding model to orbital shells, our picture of the atom has changed a lot over time.
Our ideas about the structure of the atom have changed and developed over the last 150 years.
The diagram shows one of the early models, which was proposed by the scientist J.J Thomson in 1904. We call it the "plum pudding model". Plum puddings were a very popular English dessert at the time.
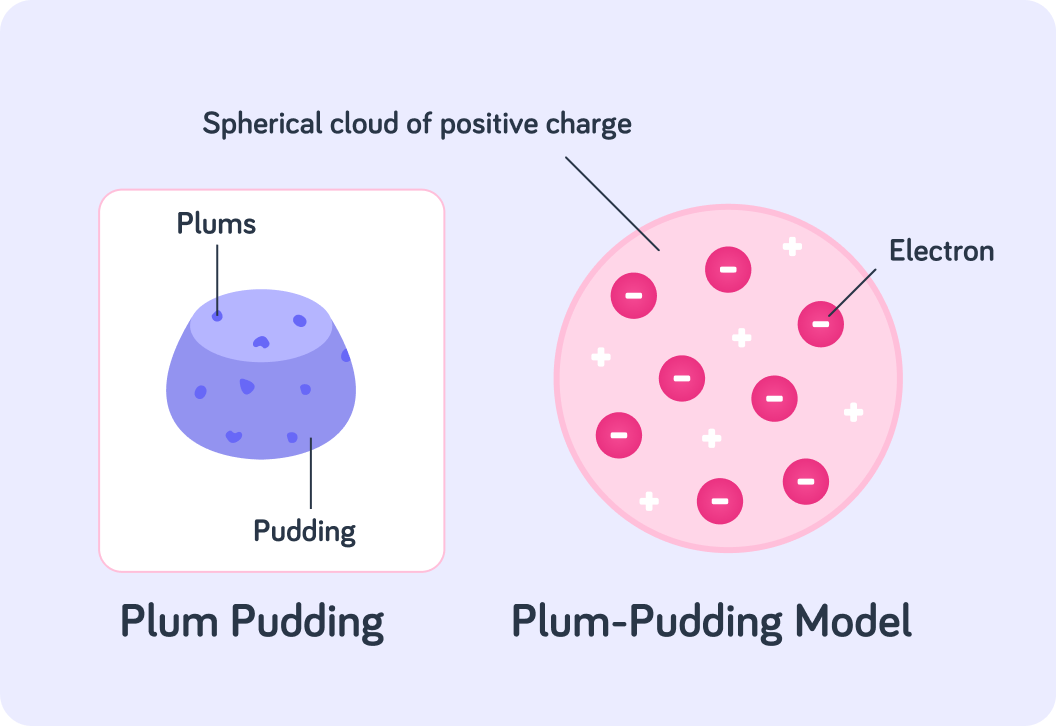
A plum pudding had plums randomly distributed throughout the ball of the pudding. Which subatomic particles do you think the plums represent in the plum pudding model?


J.J Thomson was in fact the one who discovered electrons.
Before this, scientists thought that atoms were simply solid spheres without any subatomic particles.

Think about the model of the atom we use today. **** How is the plum pudding model different today's model of the atom? Pick all the options you think are correct.

You can select multiple answers
According to the plum pudding model, an atom was a spherical cloud of positive charge with randomly distributed electrons. However, this model was proposed way back in 1904, so there was still a lot to be discovered about the structure of the atom.
The next big development in our understanding about the structure of the atom happened in 1911. This was the year that a scientist named Ernest Rutherford carried out a very important experiment.
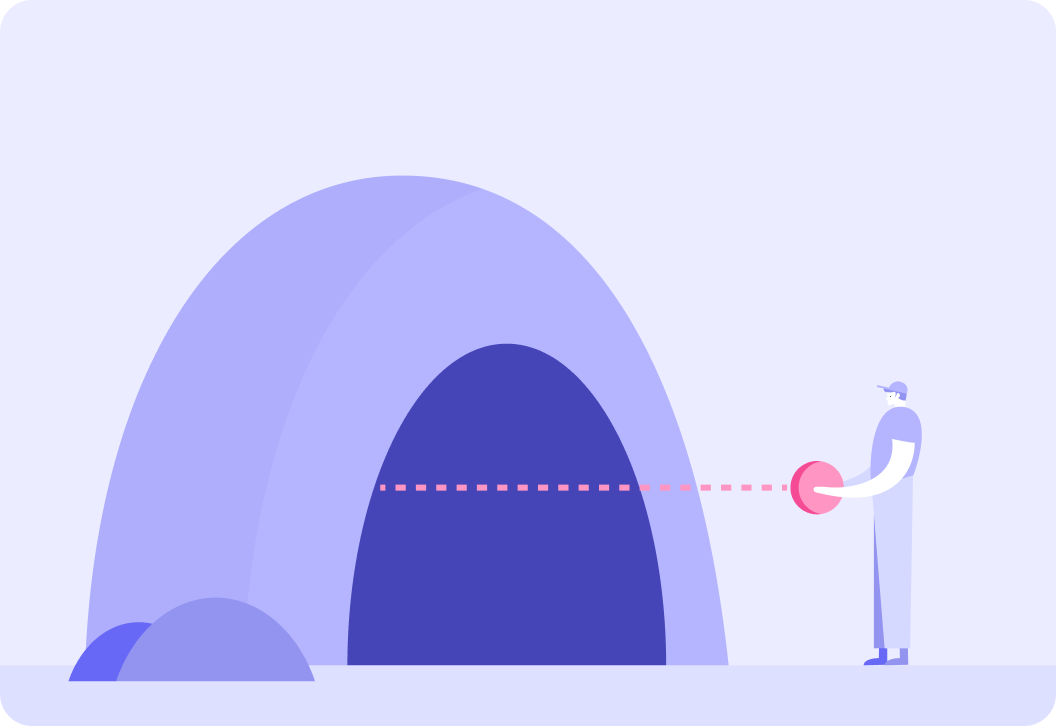
Imagine you are standing in front of the entrance to a completely dark cave, that you don't really want to go in. However, you do want to get an idea of the shape of the cave on the inside. If you throw a ball straight into the cave and the ball bounces back, what can you conclude?
A) The cave must be circular. B) There must be someone in there who threw the ball back. C) The ball must have hit the back wall of the cave.
Answer A, B or C.

You could keep throwing balls into the cave at different angles.
By looking at which balls came back at which angles (and which balls didn't come back at all), you could start to get an idea of the size of the cave and the obstacles the balls hit.
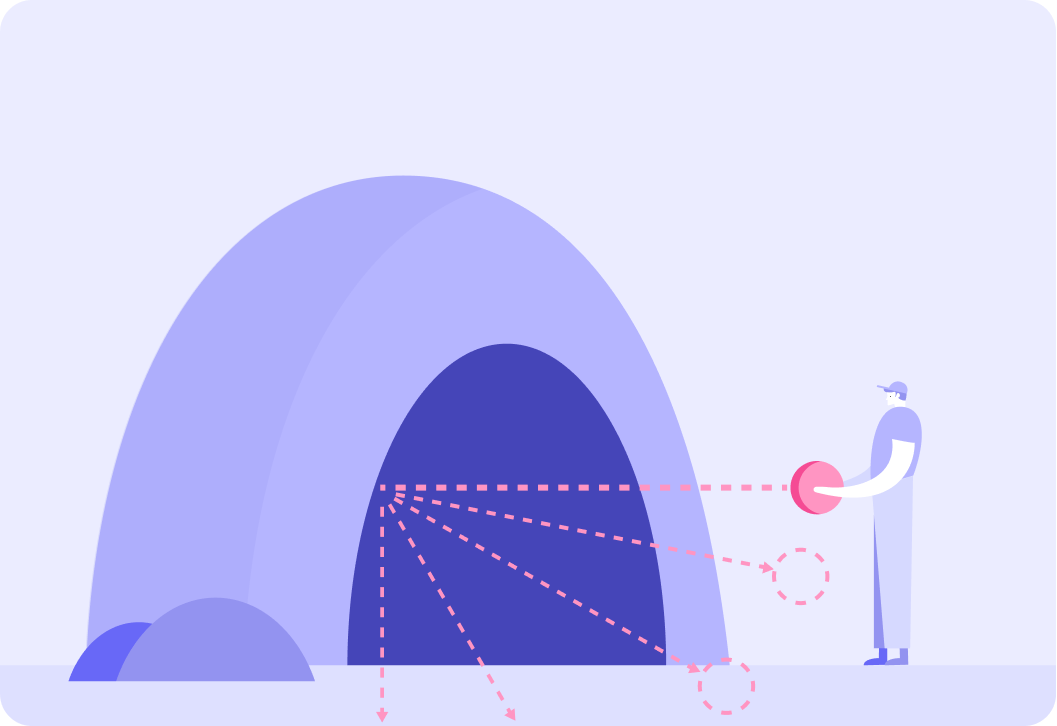
The cave example is similar to what Rutherford did to find out about the structure of the atom.
He bombarded gold atoms with alpha particles, and observed what happened to the alpha particles when they reached the atoms. The diagram shows the experiment set up.
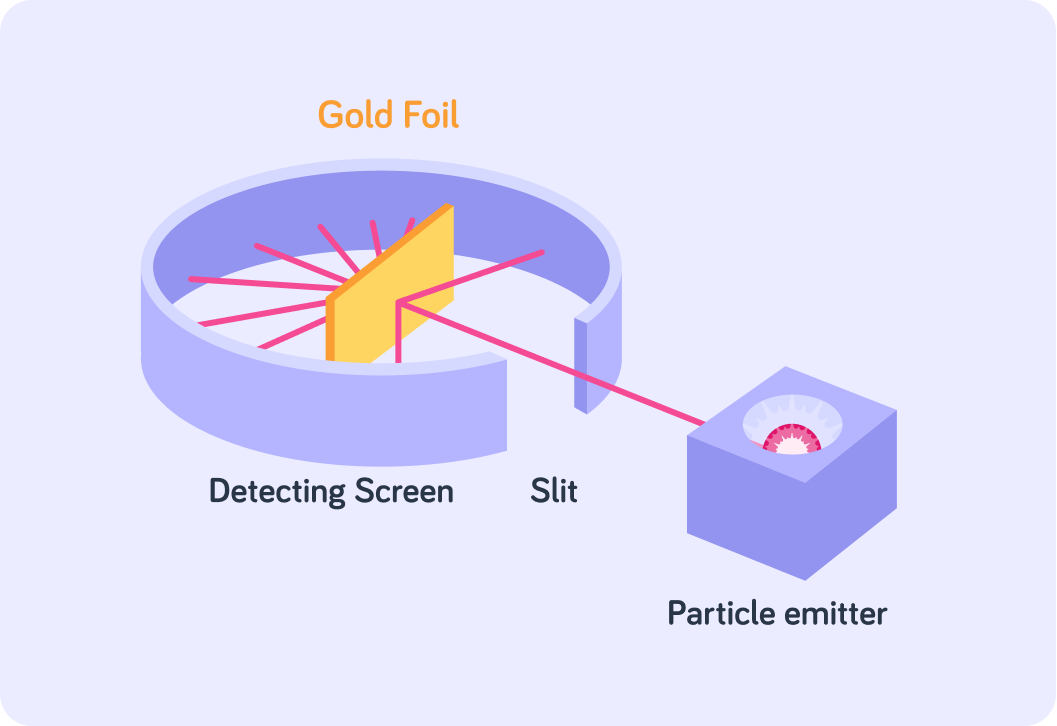
Alpha particles are tiny particles. They are made up of 2 protons and 2 neutrons. What charge do they have?


Rutherford fired these tiny tiny particles at a piece of gold foil.
It was very important that the gold foil was very very thin!

At this time, scientists thought the atom a spherical cloud of positive charge with electrons scattered randomly inside it (like a plum pudding). What do you think Rutherford expected the alpha particles to do when they hit the gold atoms?
A) Pass straight through B) Reflect back C) Scatter at different angles
Answer A, B or C.


Rutherford believed that the plum pudding model was accurate, so he expected the alpha particles to pass straight through the gold atoms and therefore straight through the gold foil.
He believed that the majority of the atom was simply a cloud of positive charge (not solid), with no areas of concentrated charge that could repel the alpha particles.

Do you think Rutherford saw the results he was expecting when he ran his experiment? Answer yes or no.


The first image shows the expected results of the experiment with the alpha particles passing straight through the atoms, and the second picture shows what actually happened.
Rutherford observed that most alpha particles passed straight through, but he also observed that some were reflected and some were deflected.
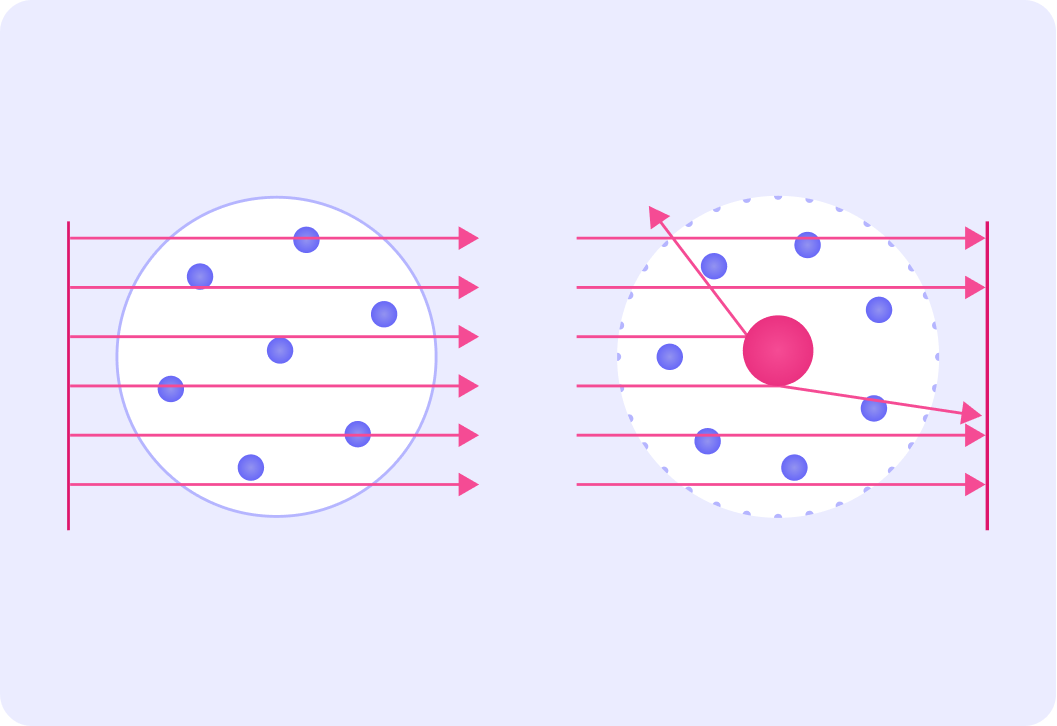
So Rutherford found that the vast majority of particles passed straight through the gold atoms. So what do you think Rutherford learned about the structure of atoms?
A) An atom is mostly solid. B) An atom is mostly empty space. C) An atom is mostly positively charged.
Answer A, B or C.


What do you think Rutherford learned from the fact that a small number of positive alpha particles were reflected from the gold atoms?
A) An atom has no positive charge. B) The positive charge of an atom is concentrated to its centre. C) Electrons are concentrated to its centre.


Rutherford realised that the positive charge of an atom must be concentrated in its centre.
He was able to conclude this because the few particles that were repelled and reflected were at a particular angle from the centre.

A small number of alpha particles changed direction somewhat without being fully reflected back - they were deflected.
Rutherford concluded that they came close enough to the positively charged centre of the atom for their pathway to be altered, but not close enough to be reflected.

So after his alpha particle scattering experiment, Rutherford proposed the new idea that most of the atom was empty space with a positively charged nucleus. What do you think happened next?

Just a few years later in 1913, another scientist, Niels Bohr, contributed further to the model of the atom. What do you think he discovered that hadn't already been discovered by the alpha scattering experiment or the plum pudding model?

Bohr put forward an atomic structure that introduced stable stationary orbits for the electrons, each one being at discrete distance from the nucleus. What do you think this model is sometimes known as?

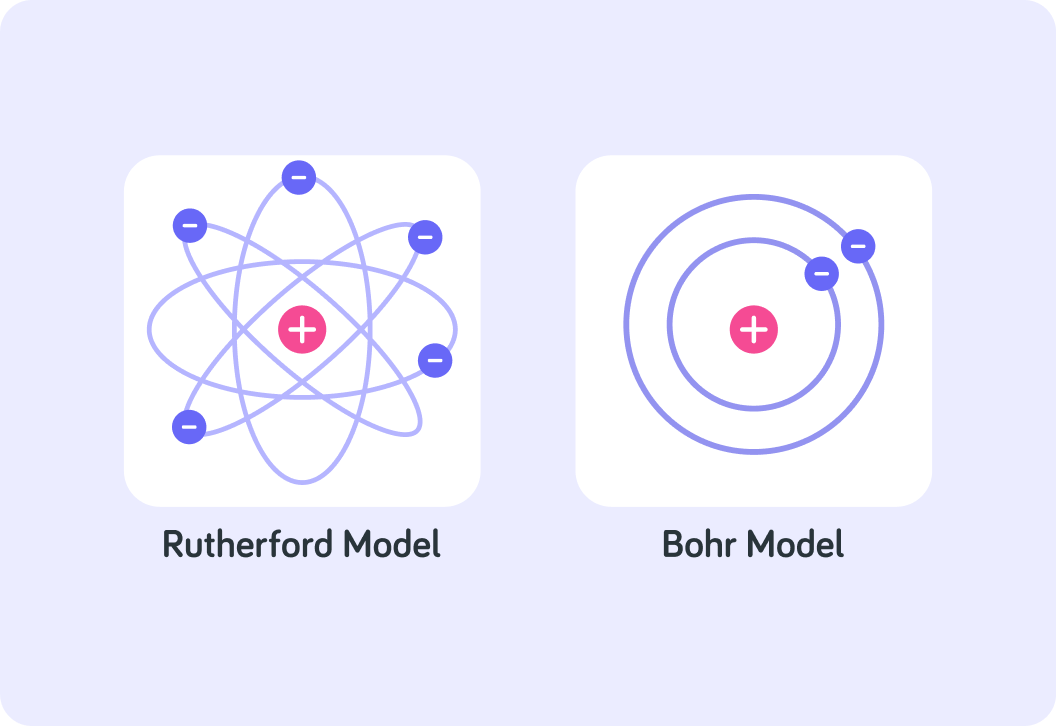
This image shows the model of the atom which Rutherford proposed after his gold foil experiment, compared with Bohr's new idea.
Notice that the centre is just a solid positive charge and electrons orbit around it. Bohr's model simply introduces stable orbits for the electrons, each at a particular distance to the nucleus.
Because of the work done by Rutherford and Bohr, the accepted model of the atom in 1913 was very similar to our model today, but what was yet to be discovered?

In 1932, another important scientist named James Chadwick did some experiments and discovered neutrons. What do you think he found out in his experiments? Pick all the options you think are correct.

You can select multiple answers
So we can thank Thomson, Rutherford, Bohr and Chadwick for the model of the atomic structure that we have accepted today.
