YOU ARE LEARNING:
What the Atomic Model Is
What the Atomic Model Is
Atoms are made up of positive protons, neutral neutrons and negative electrons.
All matter is made up of tiny atoms, and atoms themselves are made up of even smaller subatomic particles. What are these subatomic particles called?

Here is a diagram of the structure of a lithium atom. How many protons does it have?

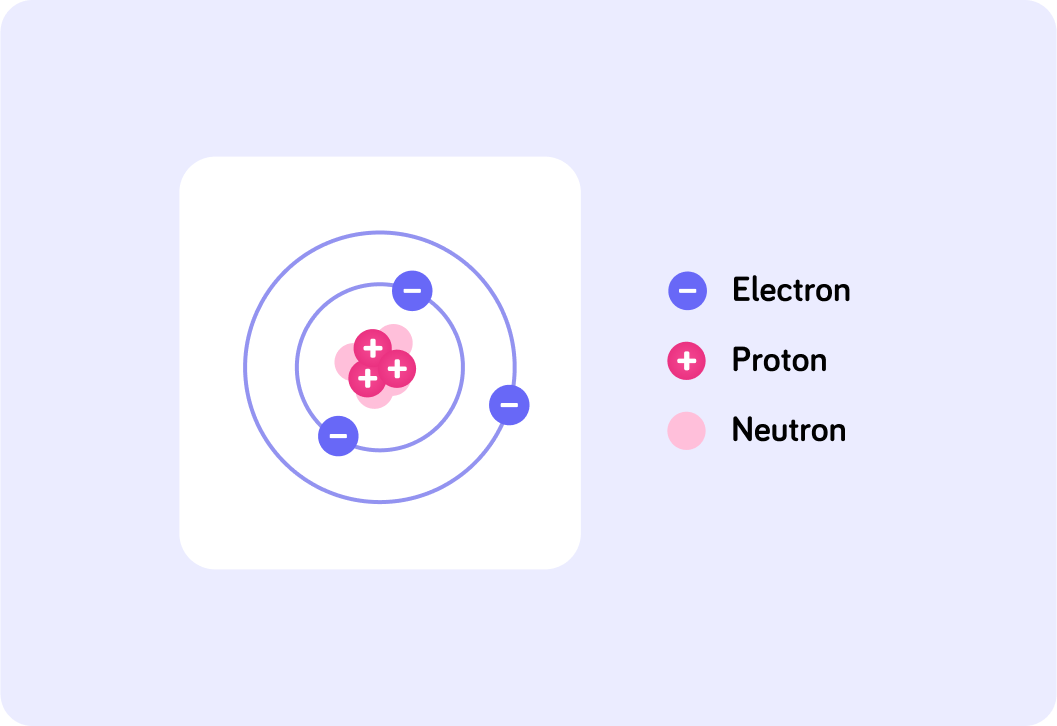
All lithium atoms have 3 protons.
This is why it has an atomic number of 3 on the periodic table.

How many electrons does the lithium atom have?


The number of protons and electrons in the lithium atom is ________.


What charge do subatomic particles have?
You can remember the charge of a proton and neutron by linking the first letter of their name, to the charge they have, so a proton has a __________ charge.

If a proton has a positive charge, then a neutron has a _______ charge.

If protons are positive and neutrons are neutral, what charge do you think an electron has?

When talking about the charges of subatomic particles, we usually think about them in relation to each other, so a proton has a relative charge of +1, and a neutron a relative charge of 0. What do you think the relative charge of an electron is?

An atom has an overall net charge of zero. In other words, it's neutral. Why do you think that is?

Look at the lithium atom again. If electrons have an insignificant mass, then where do you think most of the atoms mass is concentrated?


We know that the atom itself has zero net charge, but what charge do you think the nucleus of an atom has? Keep in mind that the nucleus has only protons and neutrons in it.


Although an atom has an overall net charge of zero, the nucleus of an atom always has a positive charge.
This is because the protons in the nucleus have a positive charge, and neutrons have no charge at all.

Atoms which undergo ionic bonding must have opposing charges. If protons are positive, electrons are negative, and neutrons are neutral, how can an atom achieve an overall positive charge?

The atomic number or proton number is what defines an element.
For example, lithium has an atomic number of 3, so all lithium atoms have 3 protons. If it lost a proton, it would become helium! Helium has an atomic number of 2.
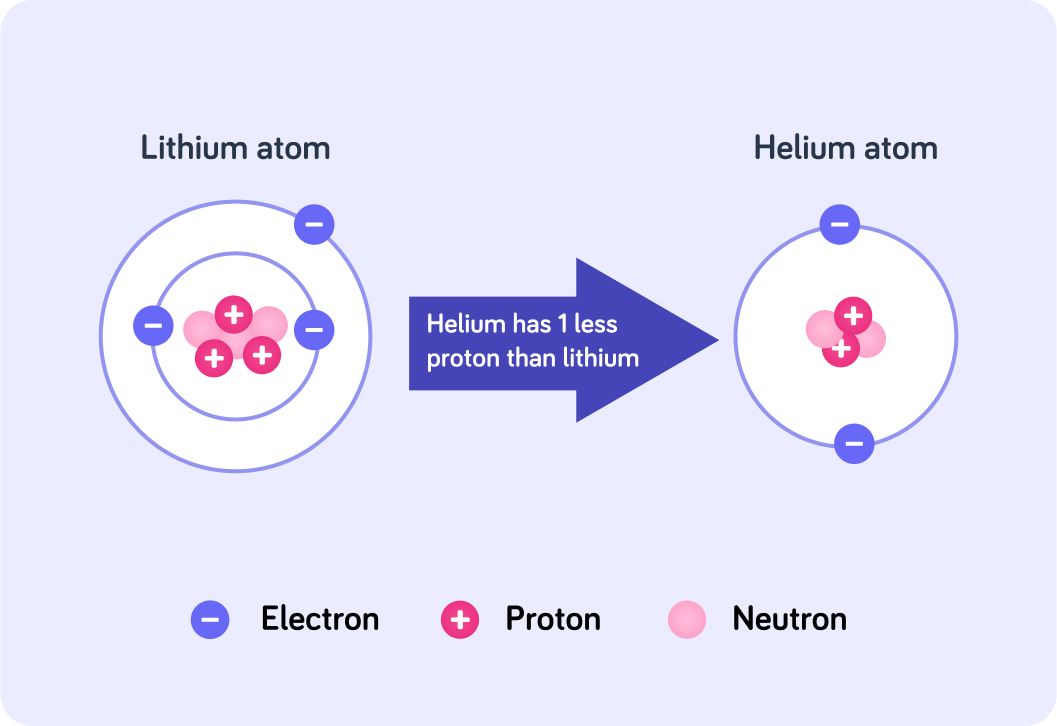
The number of protons in an atom does not change under normal conditions.
Protons can only be only lost or gained in radioactive decay, or nuclear reactions.

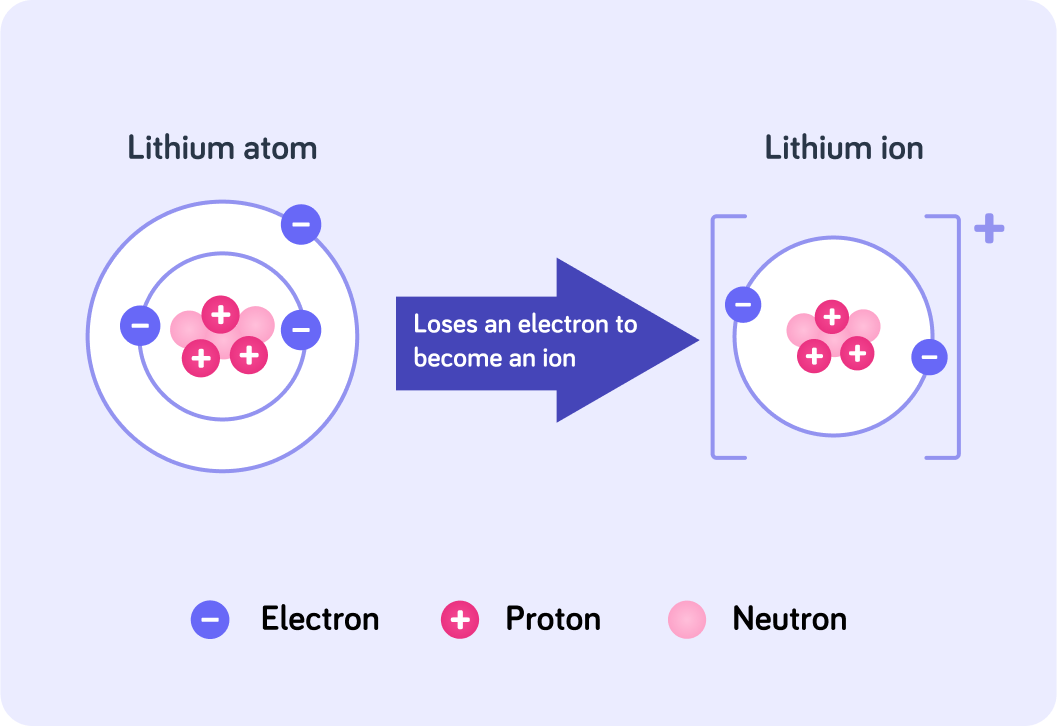
Atoms can gain or lose electrons much more easily. When an atoms loses or gains an electron, it becomes a charged particle - an ion.
For example, lithium can lose an electron to become a positively charged ion.
This is an atom of carbon, and its notation in the periodic table. What do you think the atomic number of carbon is?


Carbon's atomic number is 6.
This means that all carbon atoms have 6 protons.

The relative atomic mass of a carbon atom is 12.0.
The atomic mass of an atom is the total number of protons and neutrons. Carbon has 6 protons and 6 neutrons, so its atomic mass is 12.

If the atomic mass is the number of protons and neutrons an atom has, then where is most of the mass of an atom concentrated?


The number of electrons an atom has doesn't affect its atomic mass, because the mass of an electron is so small that it is negligible. If the relative mass of a proton is 1, and a neutron is 1, what do you think is the relative mass of an electron?

The relative mass of an electron is only about 0.0005, so very very little!
Atoms are tiny, but the nucleus of an atom is extremely tiny. In fact if an atom was the size of a football stadium, the nucleus would be the size of a marble! Which option seems the most likely proportion of the radius of a nucleus, to the radius of an atom?

Atoms and their nuclei are so tiny that it is easier to give their size using standard form. For example, an atom of copper has a diameter of 0.000000000128 metres. It's much easier to write its diameter as 1.28×10−10m .
This diagram compares some different objects and the order of magnitude of their size.
The exact diameter is different in atoms of different elements, but they are all around 10−10m.

We say that atoms have an order of magnitude of 10−10.
An order of magnitude simply approximates a number to the nearest power of 10.

If you know that atoms have an order of magnitude of 10−10, what do you think the is most likely order of magnitude of the nucleus of an atom?
A) 10−5 B) 10−10 C) 10−14
Answer A, B or C.


